Figure 1. CSR model for IgG1 production at the mouse Igh locus.
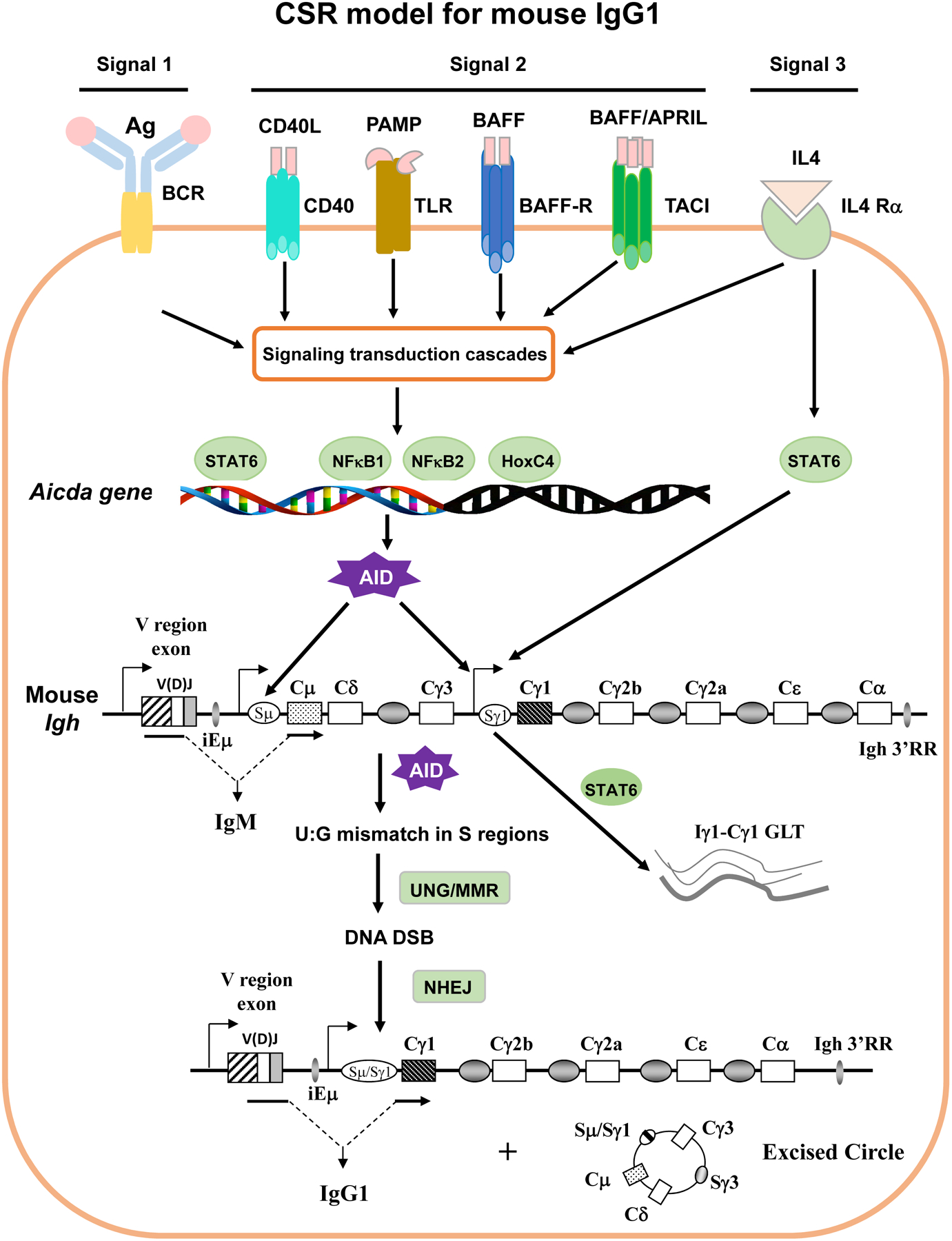
Antigen-specific antibody responses are mediated by stimulating multiple receptors expressed on B cells with their cognate ligands. The signals emanating from these receptors can be categorized into three major types. Signal 1 is the initiating signal generated by BCR upon recognizing antigen. Signal 2 is generated by co-receptors (CD40, TLRs, etc.) upon recognizing their individual ligands. Signal 3 is generated by cytokine receptors (e.g., IL-4R) upon binding to specific cytokines. Signals originating from different receptors are transduced through signaling cascade and eventually lead to activation of different transcription factors (e.g., NF-κB, HoxC4 etc). The activated transcription factors induce the expression of AID and the Igh GLT (e.g., Cγ1 GLT) that allows AID to access Sγ1 region. The genomic configuration of the rearranged mouse Igh locus is shown. AID introduces point mutations into variable (V) region exon during SHM (not depicted). During CSR, AID initiates U:G mismatches in the donor Sμ and the downstream acceptor Sγ1 regions. AID-initiated U:G mismatches are processed and converted into DNA double strand breaks (DSBs) by UNG and mismatch repair (MMR) pathways. Broken S regions are rejoined via non-homologous end-joining (NHEJ), while intervening DNA is excised as a circle. Transcription is required for both SHM/CSR with promoters delineated for both V and S regions (arrows). Upon CSR, originally expressed Cμ exons are replaced by Cγ1 exons so that naïve IgM+ B cells switch to antigen experienced IgG1+ B cells (See details in text).
