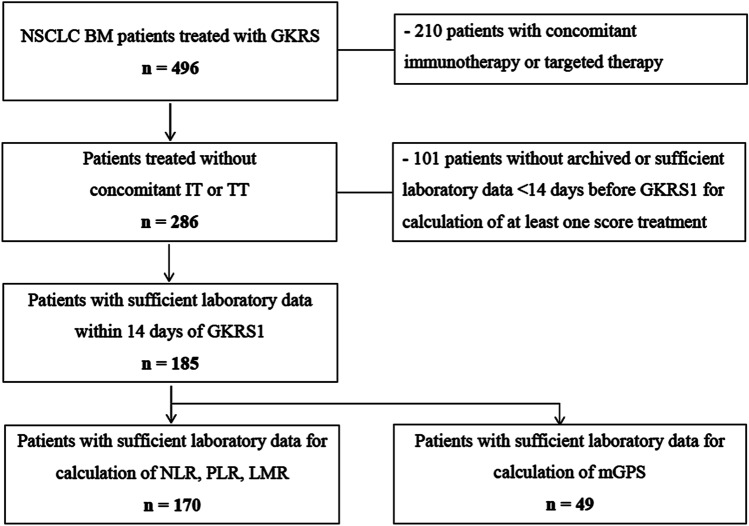
Fig. 1.
Flow chart depicting study inclusion algorithm. Between 2012 and 2018, 496 NSCLC patients with brain metastases (BM) were treated with GKRS. To evaluate the prognostic scores in a selected group of IT or TT naïve BM-NSCLC patients, 210 patients with concomitant immunotherapy or targeted therapy at (± 30 days) or after GKRS1 were excluded from the study. By excluding patients with previous or concomitant IT or TT, the prognostic scores could be evaluated in 286/496 (58%) patients. However, 101/286 (35%) patients without archived or sufficient laboratory data < 14 days before GKRS1 for calculation of at least one score had to be excluded. In total, 185/286 (65%) patients with sufficient laboratory data could be evaluated. Indeed, the baseline characterstics of the 185 included patients did not show any significant differences to the 101 excluded patients. The majority (170/185, 92%) of patients had available pre-treatment values for the calculation of NLR, PLR and LMR. However, due to missing albumin values, the mGPS could only be evaluated in 49/185 (26%) patients. BM brain metastases, GKRS Gamma Knife Radiosurgery, IT immunotherapy, LMR Lymphocyte-to-Monocyte-Ratio, mGPS modified Glasgow Prognostic Score, NLR Neutrophil-to-Lymphocyte Ratio, NSCLC non-small-cell lung cancer, PLR Platelet-to-Lymphocyte-Ratio, TT targeted therapy