FIGURE 2.
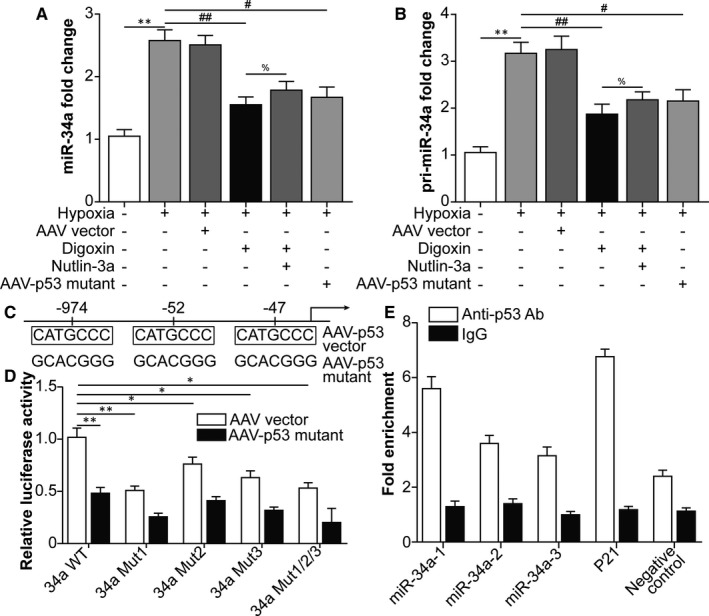
Hypoxia‐inducible factor‐1α –dependent p53 activation promotes miRNA‐34a expression in ARPE‐19 cells following hypoxia. Human RPE cells were divided into the following groups: normal, hypoxia, hypoxia + AAV vector, hypoxia + digoxin, hypoxia + digoxin + nutlin‐3a (MDM2 inhibitor and p53 agonist; 5 μmol/L for 24 h) and hypoxia + AAV‐p53 mutant (S15A, S20A and S46A) infection. A, qRT‐PCR was performed to measure miRNA‐34a levels in ARPE‐19 cells. B, qRT‐PCR was performed to measure pri‐miRNA‐34a levels in ARPE‐19 cells. In Figure 2A,B, ** P < .01, hypoxia group vs normal group. ## P < .01, # P < .05, compared with the hypoxia group. % P < .05, hypoxia + digoxin + nutlin‐3a group vs hypoxia + digoxin group. C, The p53‐binding sites in the miRNA‐34 promoter. D, P53 regulated the miRNA‐34a promoter. The p53 mutant resulted in a decrease in miRNA‐34a promoter activity (miRNA‐34a WT). The mutagenesis of the three p53‐binding sites, singularly (miRNA‐34a mutant1, miRNA‐34a mutant2 and miRNA‐34a mutant3) or in combination (miRNA‐34a mutant1/2/3), abrogated this effect. E, ChIP was conducted with an anti‐p53 antibody on ARPE‐19 genomic DNA. The immunoprecipitated chromatin was found to be enriched with the target miRNA‐34a promoter (miRNA‐34a‐1, miRNA‐34a‐2 and miRNA‐34a‐3, the regions encompassing each of three p53‐binding sites) by qPCR. Data are reported as fold enrichment over control samples (immunoprecipitation with IgG)
