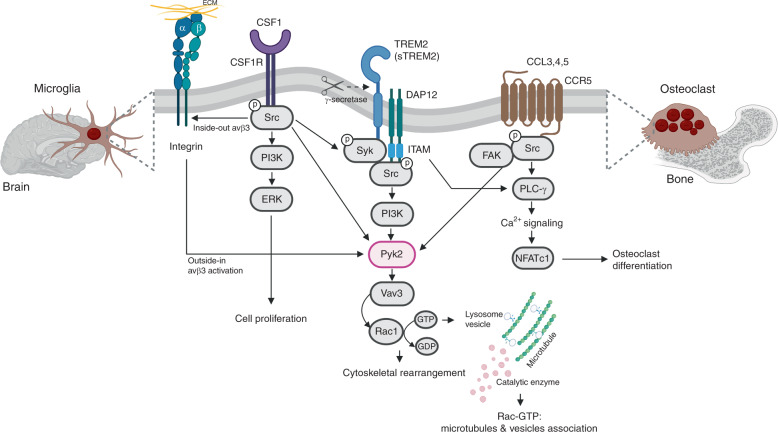
Fig. 2.
TREM2/DAP12-, CSF1/CSF1R-, and CCR5 pathways in osteoclasts and microglia. Once ligands bind to TREM2, two tyrosine residues in the immunoreceptor tyrosine-based activation motif (ITAM) of DAP12 are phosphorylated, which in turn recruits Syk kinase to activate downstream molecules such as Src tyrosine kinase and phosphatidylinositol 3-kinase (PI3K). The soluble form of TREM2 (sTREM2) is generated by ɣ-secretase, which activates PI3K, extracellular signal-regulated protein kinase (ERK), and NF-kB. Src, the main effector of CSF1R, is a tyrosine kinase that phosphorylates the ITAM tyrosine residues. CCL3/4/5 binding to CCR5 activates G protein and multiple downstream signals such as Src, PLC-ɣ and PI3K. PI3K triggers the tyrosine phosphorylation of focal adhesion complex components such as Pyk2, paxillin, Crk, and p130Cas, leading to interaction with Vav. Pyk2-Vav interaction may control Rho family GTPase activation, thereby driving localized actin polymerization that stabilizes contacts with matrix and/or promote migration