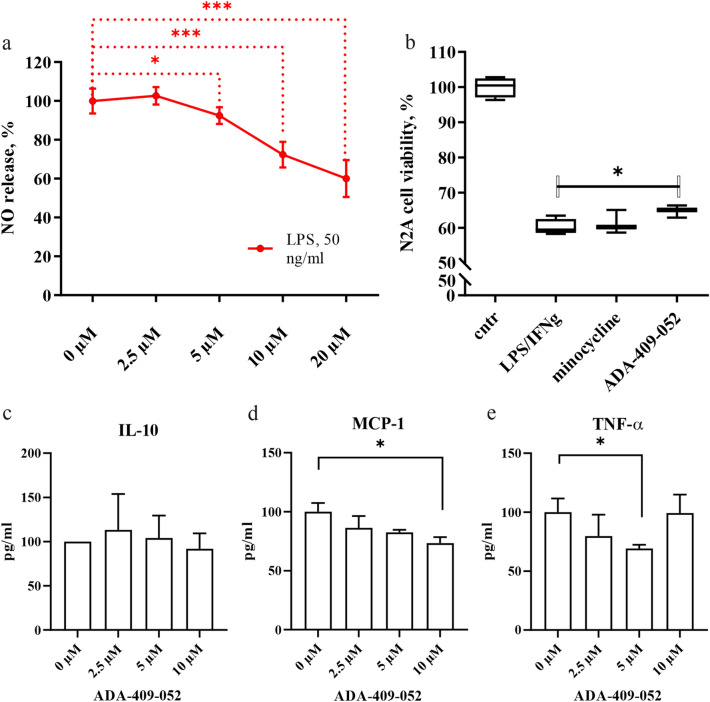
Figure 4.
(a) ADA-409-052 inhibits LPS-induced inflammation in BV2 microglial-like cells. The addition of 5, 10, and 20 µM ADA-409-052 significantly attenuated the release of NO, measured from the media of LPS-exposed BV2 cells using the Griess reagent, when compared to cells treated with LPS only. Data are represented as percentages of NO-release after normalization to LPS-stimulated BV2 cells ± s.e.m.; unpaired, two-tailed student’s t-test; technical n = 6; *p < 0.05, ***p < 0.0001. (b) ADA-409-052 protects N2a neuronal cells when co-cultured with LPS-stimulated RAW 264.7 macrophages. Boxplots show that LPS and INF-γ treatment of co-cultured N2a cells and RAW 264.7 macrophages decreased the viability of N2a cells about 40% as detected by the CytoFLEX S (Beckman Coulter) as a fraction of CD11b negative (CD11b-), PI negative (PI-), surviving cells to the total number of gated cells, normalized to the control sample. Co-treatment of ADA-409-052 and LPS/INF-γ resulted in a significant increase of the N2a cell viability, detected by the reduced percentage of CD11b-/ PI positive (PI+, dead) cells when compared to LPS/INF-γ treatment only. In contrast, the proportion of CD11b-/PI+ N2a cells remained unaltered by minocycline. Cell survival is displayed as individual boxplot per treatment with interquartile range, whiskers set from min to max; unpaired, two-tailed student’s t-test; technical n = 3/4; *p = 0.0314. (c–e) ADA-409-052 alters the expression of pro- and anti-inflammatory cytokine expression of BV2 microglial cells. By measuring cytokine-secretion of ADA-409-052-stimulated BV2 cells, using the BD CBA mouse inflammation kit, (c) no changes in secretion of the anti-inflammatory IL-10 were detected. (d) However, we detected a major decrease of MCP-1 upon the addition of 10 µM ADA-409-052. (e) Furthermore, TNF-α decreased upon ADA-409-052-stimulation (5 µM) significantly when compared with untreated cells (0 µM ADA-409-052). Student’s t-test, n = 4; *p < 0.05.