Abstract
Increased activity and excitability (sensitisation) of a series of molecules including the transient receptor potential ion channel, vanilloid subfamily, member 1 (TRPV1) in pain-sensing (nociceptive) primary sensory neurons are pivotal for developing pathological pain experiences in tissue injuries. TRPV1 sensitisation is induced and maintained by two major mechanisms; post-translational and transcriptional changes in TRPV1 induced by inflammatory mediators produced and accumulated in injured tissues, and TRPV1 activation-induced feed-forward signalling. The latter mechanism includes synthesis of TRPV1 agonists within minutes, and upregulation of various receptors functionally linked to TRPV1 within a few hours, in nociceptive primary sensory neurons. Here, we report that a novel mechanism, which contributes to TRPV1 activation-induced TRPV1-sensitisation within ~ 30 min in at least ~ 30% of TRPV1-expressing cultured murine primary sensory neurons, is mediated through upregulation in cyclooxygenase 2 (COX2) expression and increased synthesis of a series of COX2 products. These findings highlight the importance of feed-forward signalling in sensitisation, and the value of inhibiting COX2 activity to control pain, in nociceptive primary sensory neurons in tissue injuries.
Subject terms: Neuroscience, Peripheral nervous system
Introduction
Tissue injuries are followed by an inflammatory reaction, which aims to restore tissue integrity1. This inflammatory process is associated with pathological pain experiences including hypersensitivity to mechanical and heat stimuli known respectively as mechanical allodynia and heat hyperalgesia in humans2–4. The non-selective cationic channel, transient receptor potential ion channel, vanilloid subfamily, member 1 (TRPV1) is expressed in a major group of pain-sensing (nociceptive) primary sensory neurons, the first neurons detecting tissue pathology—it plays a pivotal role in the development of heat hyperalgesia and significantly contributes to the development of mechanical allodynia in inflammation following tissue injury5,6.
The development of hypersensitivities depends on plastic changes, known as sensitisation (a use-dependent increase in the activity and excitability), in neurons involved in nociceptive processing7. Upregulation of TRPV1 expression, TRPV1 translocation from the cytoplasm to the cytoplasmic membrane and post-translational modification-mediated increases in TRPV1 responsiveness and activity significantly contribute to the sensitised state of nociceptive primary sensory neurons and underlay the pivotal role of TRPV1 in the development of hypersensitivities to heat and mechanical stimuli in tissue inflammation8–17. Inflammatory mediators including nerve growth factor, bradykinin, prostaglandins (PGs) and ligands of various protease activated receptors (PARs), such as thrombin, mast cell-derived tryptase or kallikrein acting on their cognate receptors on TRPV1-expressing primary sensory neurons, initiate those changes in TRPV110,12,13,18–20. In addition, TRPV1 activation itself may also increase its own responsiveness and activity through feed-forward mechanisms occurring at various levels of signalling in primary sensory neurons. Firstly, TRPV1 activation through Ca2+ influx induces the synthesis of the TRPV1 endogenous ligand anandamide in TRPV1-expressing primary sensory neurons within minutes21,22. Secondly, TRPV1 activation also induces, within hours, upregulation of the expression of genes whose products are implicated in post-translational modification-mediated increases in the responsiveness and activity of TRPV123. Previously, Zhang and colleagues have reported a third type of feed-forward TRPV1 sensitisation that develops within ~ 20–30 min following TRPV1 activation by the archetypical exogenous ligand capsaicin and is mediated through extracellular signal-regulated kinase 1/2 (ERK1/2) and the Ca2+-calmodulin-dependent protein kinase II (CaMKII) activity24. Here, we show that this third type of feed-forward TRPV1 sensitisation depends on upregulation of cyclooxygenase 2 (COX2) and the subsequent increase in the synthesis of a series of PGs.
Results
Capsaicin induces sensitisation of responses to subsequent capsaicin application in ~ 30 min in mouse cultured primary sensory neurons
Responses to capsaicin were assessed by measuring changes in the intracellular Ca2+ concentration. The most consistent development of sensitised responses, to the second capsaicin application was observed when 500 nM capsaicin, which is about the EC50 of this compound at mouse TRPV15, was applied for 15 s, 30 min before the second capsaicin (500 nM) application. The 30-min superfusion of the cells with the extracellular buffer however, resulted in a significant reduction in KCl-evoked responses indicating a general “run down” of the responses (p < 0.0001, Student’s t-test, n = 288; Fig. 1A–C; Supplementary Fig. 1). Therefore, the amplitude of each capsaicin-evoked response was normalised to the amplitude of the subsequent KCl-evoked response. Then, the first and second normalised capsaicin-evoked responses were compared. This comparison revealed that, in 84 of the 288 capsaicin-responding neurons, the second normalised capsaicin-evoked response was more than 5% greater than the first response (Fig. 1A,B). These cells were considered sensitizer neurons. All other capsaicin-responsive neurons (n = 204) were considered as “non-sensitizer” (Fig. 1C). Within the 84 sensitizers, three cells were considered outliers; change in the intracellular Ca2+ concentration during the first capsaicin application remained under the threshold value in these cells. However, the second application induced an appropriately timed and characteristic change in the intracellular Ca2+ concentration that was well above the threshold value. Nevertheless, due to the lack of a proper first response, the ratio of the second and first normalised capsaicin-evoked responses of these cells significantly exceeded (11.84, 20.63 and 120.41) the average of the 84 cells (3.035 ± 2.44). Based on their overall properties, these three cells were not included in the statistical analysis of the control recordings.
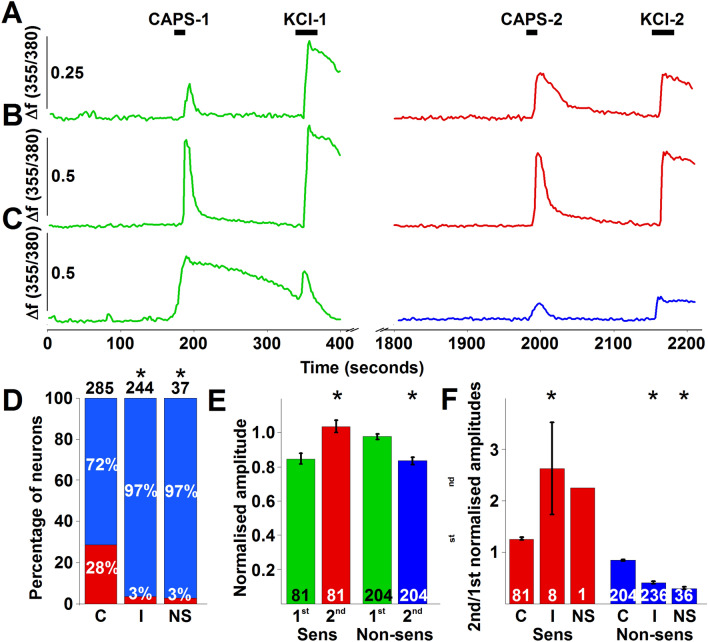
Figure 1.
Capsaicin application to cultured murine primary sensory neurons induces sensitised responses to capsaicin applied 30 min after the first application in a group of neurons in a COX2-dependent manner. (A–C) shows typical recordings of changes in the intracellular Ca2+ concentrations in three cultured murine primary sensory neurons in control extracellular buffer. Capsaicin (500 nM) was applied for 15 s at 3 (CAPS-1) and 33 min (CAPS-2). Applications of capsaicin were followed by the applications of KCl (50 mM) 2 min after capsaicin applications (KCl-1 and KCl2). The neurons whose responses are shown in (A,B) exhibited absolute (A), or relative to the KCl-evoked response (B), sensitised second capsaicin-evoked responses when the amplitudes are assessed. The cell whose response is shown in (C) exhibited a non-sensitised response. (D) Relative number of neurons exhibiting sensitizer (red) or non-sensitizer (blue) phenotype in control extracellular buffer (C), and in the presence of indomethacin (10 μM; I) or NS-398 (3 μM; NS). Both indomethacin and NS-398 significantly reduced the proportion of sensitizers (p < 0.0001; Fischer’s exact test for indomethacin and p = 0.0002; Fischer’s exact test for NS-398). The numbers above the columns indicate the total number of capsaicin responding neurons. (E) Averaged normalised amplitudes (to the amplitudes of KCl-evoked responses) of first (green) and second (red for sensitizers (Sens), blue for non-sensitizers (Nonsens)) capsaicin application-evoked responses. In sensitizers and non-sensitizers the second capsaicin-evoked responses were, respectively, significantly greater (p < 0.0001; Student’s paired t-test; n = 81) and smaller (p < 0.0001, Student’s paired t-test; n = 204) than the amplitudes of the first capsaicin-evoked responses. The numbers at the bottom of the columns indicate the number of neurons. (F) Ratio of first and second normalised (to KCl-evoked responses) capsaicin-evoked responses in control buffer (C) and in the presence of indomethacin (10 μM; I) or NS398 (3 μM; NS) in sensitizer (Sens) and non-sensitizer (Non-sens) neurons. Both indomethacin and NS-398 increased or reduced the ratio in sensitizer or non-sensitizer neurons, respectively (indomethacin: p < 0.0001; Student’s t-test for sensitizers and p < 0.0001; ANOVA followed by Tukey’s post-hoc test for non-sensitizers; NS398: p < 0.0001; ANOVA followed by Tukey’s post-hoc test for non-sensitizers). The numbers at the bottom of the columns indicate the number of neurons.
On average, the normalised amplitudes of the first and second responses of the 81 sensitizers were 0.85 ± 0.03 and 1.04 ± 0.04 (p < 0.0001; Student’s paired t-test), whereas those for the non-sensitizer cells were 0.98 ± 0.02 and 0.83 ± 0.02 (p < 0.0001; Student’s paired t-test; n = 204; Fig. 1E). Both the first and second average responses in the sensitizer and non-sensitizer neurons were significantly different (first responses: p = 0.0002, Student’s two samples t-test; second responses: p < 0.0001, Student’s two samples t-test). The normalised first and second responses of both the sensitizer and non-sensitizer cells exhibited correlation (R = 0.85, p < 0.0001 for the sensitizers and R = 0.77, p < 0.0001 for the non-sensitizers; Supplementary Fig. 2A).
The average ratio of the second and first normalised capsaicin-evoked responses was 1.26 ± 0.03 (n = 81) in the sensitizer, while it was 0.84 ± 0.01 (n = 204) in the non-sensitizer group (p < 0.0001, Student’s two samples t-test; Fig. 1F). Within the sensitizers, cells with smaller normalised first responses tended to show greater increase (R = − 0.49, p < 0.0001; Supplementary Fig. 2B), whereas non-sensitizers exhibited a very weak correlation between the amplitude of the first normalised responses and the ratio of the second and first responses (R = 0.21, p = 0.003; Supplementary Fig. 2B).
The development of capsaicin-induced sensitisation of subsequent capsaicin-evoked responses depends on COX2
The 30-min delay in the development of sensitised capsaicin-evoked responses following capsaicin application suggests that the sensitisation depends on changes in the transcription of immediate early genes (24; current data). A search for immediate early gene products that could induce TRPV1 sensitisation through ERK1/2 and CaMKII, the signalling molecules shown being involved24, suggested that upregulation in prostaglandin-endoperoxidase synthase 2 gene (ptgs2) and subsequent upregulation in COX2 expression and increased synthesis of COX2 products might underlie the development of capsaicin-induced sensitisation of subsequent capsaicin-evoked responses. Therefore, next we applied indomethacin (10 μM), a non-selective COX inhibitor, to the cells during the interim period between the two capsaicin applications. Indomethacin significantly reduced the proportion of cells exhibiting sensitised responses to ~ 3% (8 of 244; p < 0.0001; Fischer’s exact test; Fig. 1D). Interestingly, while the degree of sensitisation was significantly greater in the remaining sensitizers (2.63 ± 0.9, n = 8 in indomethacin and 1.26 ± 0.03, n = 81 in control; p < 0.0001; ANOVA followed by Tukey’s post-hoc test; Fig. 1F), the ratio of the second and first normalised capsaicin-evoked responses in the non-sensitizer cells was significantly smaller in the presence of indomethacin than in control (0.41 ± 0.2, n = 236 in indomethacin and 0.84 ± 0.01, n = 204 in control; p < 0.0001; ANOVA followed by Tukey’s post-hoc test; Fig. 1F).
Next, we applied the selective COX2 inhibitor NS-398 (3 μM) to the cells to specifically explore whether COX2, whose products have been reported to contribute to TRPV1 sensitisation13,19,20, is responsible for the sensitisation of the second capsaicin-evoked responses. As seen with indomethacin, NS-398 also significantly reduced the proportion of the sensitizing neurons (~ 3%, 1 of 37; p = 0.0002; Fischer’s exact test; Fig. 1D). Again, the size of sensitisation in the single sensitizer neuron appeared greater (2.24 ± 0.9, n = 1 in NS-398), whereas the ratio of the second and first normalised capsaicin-evoked responses in the non-sensitizers was significantly smaller in the presence of NS-398 than in control (0.3 ± 0.03, n = 36; p < 0.0001; ANOVA followed by Tukey’s post-hoc test; Fig. 1F). Collectively, these data suggested that capsaicin induced upregulation of ptgs2, COX2 and COX2 products could indeed significantly contribute to capsaicin-evoked sensitisation of subsequent capsaicin-induced responses in primary sensory neurons. Thus, we further tested this hypothesis.
Capsaicin application to mouse cultured primary sensory neurons induces ptgs2 and COX2 upregulation
Conventional RT-PCR was used to assess ptgs2 expression in cultured primary sensory neurons after treating them with capsaicin or buffer for control. The size of the RT-PCR product (214 bp) was indistinguishable from the predicted size (Fig. 2A; Supplementary Fig. 3). Analysis of gel images revealed that capsaicin treatment indeed significantly upregulated ptgs2 expression (p = 0.0004; Student’s two samples t-test, n = 3) in cultured primary sensory neurons (Fig. 2A,B).
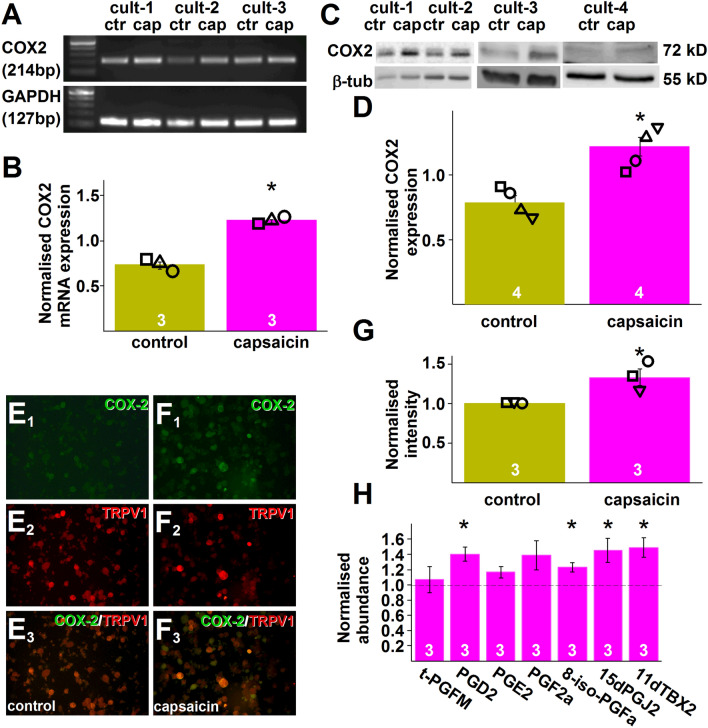
Figure 2.
Capsaicin application to cultured murine primary sensory neurons upregulates COX2 expression and synthesis of a series of COX products. (A) Gel image of RT-PCR products amplified using primer pairs for murine ptgs2 (COX2) and gapdh (GAPDH) and cDNA prepared using RNA isolated from three cultures (cult-1–cult-3) of murine primary sensory neurons 25 min after incubating the cells in control (ctr) buffer or in the presence of 500 nM capsaicin (cap) for 5 min. (B) Averaged normalised ptgs2 expression in cultures shown in (A). Capsaicin (500 nM, capsaicin) application resulted in a significant increase in ptgs2 expression (p = 0.0004; Student’s t-test, n = 3). (C) Gel images of immunoblots prepared using proteins extracted from four cultures (cult-1–cult-4) of murine primary sensory neurons 25 min after incubating the cells in control (ctr) buffer or in the presence of 500 nM capsaicin (cap) for 5 min and antibodies raised against COX2 and GAPDH. (D) Averaged normalised COX2 expression in cultures shown in (C). Capsaicin (500 nM, capsaicin) application resulted in a significant increase in COX2 expression (p = 0.0025; Student’s t-test, n = 4). (E1,E2,E3,F1,F2,F3) Microscopic images of cultured murine primary sensory neurons incubated in control buffer (E1,E2,E3) or in the presence of 500 nM capsaicin (F1,F2,F3) for 5 min. Cultures were fixed 25 min after the incubation and incubated in anti-COX2 (green) and anti TRPV1 antibodies (red). (E1,F1) show COX2 staining, (E2,F2) show TRPV1 staining whereas (E3,F3) show composite image. Note that COX2 staining intensity is increased in the culture incubated in the presence of capsaicin. (G) Normalised averaged intensity values of COX2 immunostaining in three cultures of murine primary sensory neurons. Capsaicin significantly increased the staining intensity (p = 0.0337; Student t-test; n = 3). (H) Targeted UPLC-MS measurements of seven COX products in an eicosanoids panel using samples prepared from cultured murine primary sensory neurons incubated in control buffer or 500 nM capsaicin for 5 min. Samples were prepared 25 min after finishing the 5 min incubation. During the 25 min, cells were kept in the presence of the COX2 substrate arachidonic acid (10 μM). Four of the seven COX products present in the eicosanoid panel exhibited significant increase by incubating the cells in capsaicin (PGD2: q = 0.032; 8-iso-PGF2α: q = 0.028; 15dPGJ2: q = 0.046; 11dTXB2: q = 0.031; Student-t test followed by Benjamini–Hochberg FDR, n = 3).
Next, we used Western blotting and immunostaining to confirm that the de novo synthesised ptgs2 is translated into protein. Western blot images confirmed that cultured primary sensory neurons exhibit basal COX2 expression (Fig. 2C; Supplementary Fig. 4;25–28). Image analysis also revealed that capsaicin exposure induced a significant increase in that expression (p = 0.0025; Student’s t-test, n = 4; Fig. 2C,D).
Immunostaining of control cultures also confirmed basal COX2 expression in a population of neurons (Fig. 2E,F;25–28). Capsaicin exposure significantly increased the intensity of COX2 immunopositivity (p = 0.0337; Student t-test; n = 3; Fig. 2E–G). Importantly, about half the TRPV1-expressing cells exhibited COX2 immunopositivity in both conditions (Fig. 2E,F).
Capsaicin application to mouse cultured primary sensory neurons increases the concentration of a series of COX2 products
To confirm that the upregulated COX2 expression is associated with increased PG synthesis, we collected the cells and superfusate of mouse cultured primary sensory neurons following their exposure to capsaicin or buffer for control, and used ultra-performance liquid chromatography with tandem mass spectrometry (UPLC-MS) for targeted analysis of the samples for a group of arachidonic acid-derived compounds (Table 1). Our measurements showed that only a few fatty acids, lipoxygenase (LOX) products and one COX product were above the detection level in the first experiment (Table 1). Therefore, in order to increase the abundance of the products above the detection threshold, we repeated the experiments by exposing the cultures to arachidonic acid (10 µM), thus providing a substrate, during the 30-min incubation period. In these cultures, 32 of the 48 compounds included in the panel were above the detection level (Table 1). Among the COX products, four exhibited significant increase following capsaicin treatment; PGD2, 8-iso-PGFa, 15dPGJ2 and 11d-tromboxane2 (11dTBX2; Fig. 2H).
Table 1.
Concentration of 48 lipid mediators of inflammation in mouse primary sensory neuron cultures in naive condition and 30 min after incubating cells in capsaicin for 5 min (pg/ng protein).
| Without arachidonic acid | With arachidonic acid | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Average (Control) | Sem | Average (Capsaicin) | Sem | qa | Average (Control) | Sem | Average (Capsaicin) | Sem | qa | |
| C18:2 (LA) | 397.31 | 76.61 | 365.83 | 36.57 | ns | 352.25 | 32.57 | 669.5 | 55.9 | 0.029 |
| C20:3 (DGLA) | nd | nd | nd | nd | ||||||
| C20:4 (AA) | 117.3 | 5.52 | 204.56 | 54.15 | ns | 4460.15 | 1092.9 | 7646.06 | 1415.8 | 0.029 |
| C20:5 (EPA) | 8.21 | 0.62 | 11.35 | 3.85 | ns | 17.17 | 3.27 | 9.82 | 0.66 | 0.027 |
| C22:6 (DHA) | 143.48 | 8.03 | 198.12 | 51.94 | ns | 201.25 | 25.25 | 90.69 | 8.56 | 0.001 |
| 9(S)-HODE | 1.43 | 0.33 | 1.26 | 0.44 | ns | 4.06 | 0.96 | 2.78 | 0.24 | ns |
| 13(S)-HODE | 3.17 | 1.17 | 2.13 | 0.47 | ns | 4.64 | 0.55 | 3.9 | 0.5 | 0.049 |
| Tetranor-PGDM | nd | nd | nd | nd | nd | |||||
| Tetranor-PGEM | nd | nd | nd | nd | nd | |||||
| Tetranor-PGFM | 204.01 | 13.88 | 198.27 | 0.42 | ns | 172.83 | 26.46 | 175.81 | 3.57 | ns |
| 12(S)-HEPE | nd | nd | nd | 0 | nd | nd | ||||
| 15(S)-HEPE | nd | nd | nd | 0 | nd | nd | ||||
| 5,6-EET | nd | nd | 478.79 | 197.24 | 542.48 | 203.52 | ns | |||
| 8,9-EET | nd | nd | 161.04 | 39.13 | 252.32 | 54.59 | 0.042 | |||
| 11,12-EET | nd | nd | 187.74 | 45.75 | 295.21 | 68.39 | 0.028 | |||
| 14,15-EET | nd | nd | 142.32 | 43.29 | 170.7 | 60.22 | ns | |||
| 5(S)-HETE | nd | nd | 381.2 | 90.63 | 342.03 | 57.27 | ns | |||
| 8(S)-HETE | nd | nd | 167.28 | 45.16 | 153.06 | 33.58 | ns | |||
| 11(R)-HETE | 0.71 | 0.71 | 0.37 | 0.37 | ns | 250.13 | 62.74 | 246.65 | 55.09 | ns |
| 12(R)-HETE | nd | nd | 112.4 | 28.17 | 95.07 | 21.39 | ns | |||
| 15(S)-HETE | nd | 1.77 | 1.77 | ns | 390.34 | 94.73 | 296.88 | 66.49 | 0.029 | |
| 16(R)-HETE | nd | nd | 13.17 | 1.84 | 8.28 | 1.69 | 0.005 | |||
| 5,6-DHET | nd | nd | 43.21 | 11.22 | 66.13 | 13.36 | 0.031 | |||
| 8,9-DHET | nd | nd | 12.38 | 4.29 | 12.74 | 3.06 | ns | |||
| 11,12-DHET | nd | nd | 32.43 | 12.52 | 22.97 | 6.43 | ns | |||
| 14,15-DHET | nd | nd | 24.13 | 8.16 | 22.39 | 5.64 | ns | |||
| 5-Oxo-ETE | nd | nd | 163.86 | 35.67 | 186.97 | 36.17 | ns | |||
| 12-Oxo-ETE | nd | nd | 155.04 | 29.95 | 175 | 35.05 | ns | |||
| 14(S)-HDoHE | nd | nd | nd | 0 | nd | nd | ||||
| 17(S)-HDoHE | nd | nd | nd | 0 | nd | nd | ||||
| 10(S),17(S)-DiHDoHE | nd | nd | nd | 0 | nd | nd | ||||
| LTB4 | nd | nd | 10.63 | 2.41 | 7.09 | 2.04 | 0.006 | |||
| 12-Oxo-LTB4 | nd | nd | 92.44 | 24.64 | 163.49 | 41.46 | 0.044 | |||
| LTC4 | nd | nd | nd | 0 | nd | |||||
| LTD4 | nd | nd | nd | 0 | nd | |||||
| LTE4 | nd | nd | nd | 0 | nd | |||||
| PGD2 | nd | nd | 17.41 | 6.63 | 23.48 | 7.52 | 0.032 | |||
| PGE2 | nd | nd | 186.42 | 37.17 | 222.71 | 58.2 | ns | |||
| Lipoxin A4 | nd | nd | 47.54 | 10.45 | 57.75 | 15.9 | 0.045 | |||
| Lipoxin B4 | nd | nd | nd | 0 | nd | |||||
| 6-Keto-PGF1alpha | nd | nd | nd | 0 | nd | |||||
| PGF2alpha | nd | nd | 4.11 | 0.84 | 5.81 | 1.59 | ns | |||
| 8-iso-PGF2alpha | nd | nd | 3.13 | 0.55 | 3.89 | 0.82 | 0.028 | |||
| 15dPGJ2 | 1.35 | 0.06 | 1.41 | 0.27 | ns | 2.91 | 0.47 | 4.14 | 0.6 | 0.046 |
| TXB2 | nd | nd | nd | 0 | nd | |||||
| 11-dehydro TXB2 | nd | nd | 62.03 | 11.94 | 94.94 | 25.7 | 0.031 | |||
| Resolvin D1 | nd | nd | nd | 0 | nd | |||||
| Resolvin D2 | nd | nd | nd | 0 | nd | |||||
aq refers to significant differences in fold changes.
Discussion
Eicosanoids and related compounds, including PGE2, 15dPGJ2 and PGD2, PGI2 have been implicated in inducing TRPV1 sensitisation13,19,20. It is also reported that activation of primary sensory neurons leads to COX2 upregulation25–28. Further, it is reported that a group of primary sensory neurons exhibits sensitisation to capsaicin within 20–30 min after a previous capsaicin application24. However, this is the first report which shows that increased ptgs2 and COX2 expression and synthesis of a series of COX2 products are triggered by TRPV1 activation and thus underlie the development of sensitisation of TRPV1 in ~ 30 min after the first TRPV1 activation.
When capsaicin is repeatedly applied within a short period of time (i.e. tens of seconds – minutes), subsequent responses exhibit rapid desensitisation (for refs see29). However, when capsaicin application is repeated only in tens of minutes, responses are sensitised24. Here we report that, similarly to rat cultured primary sensory neurons24, murine cultured primary sensory neurons also exhibit this type of sensitisation, though there are some differences between the two species. In mouse primary sensory neurons, the estimated proportion of cells exhibiting sensitisation is lower and the correlation between the amplitude of the first and second responses is less pronounced than in rat primary sensory neurons24. Regarding the proportion of sensitizer neurons, however, it should be noted that our estimation may be conservative as only neurons which exhibited at least a 5% increase in the amplitude of the second capsaicin-evoked responses were considered sensitizers. The assumption that we underestimated the proportion of sensitizers is supported by the findings that while ~ 1/3 of the capsaicin-responsive neurons exhibited at least 5% increase in responses to the second capsaicin application, about 1/2 of the TRPV1-expressing neurons appeared to express COX2—both in the control environment and after capsaicin exposure.
While Zhang and co-workers found involvement of ERK1/2 and CaMKII signalling24 here we have identified further down-stream elements of the signalling pathway(s) which lead to capsaicin-evoked delayed sensitisation of TRPV1mediated responses. We found that blocking COX activity—either by the nonselective COX inhibitor indomethacin, or COX2 activity by the selective COX2 inhibitor NS-398—almost completely eliminated the delayed sensitisation. These data suggest that COX2 activity is pivotal in delayed TRPV1 sensitisation. However, the few sensitizer neurons remaining in our samples after blocking COX or COX2 indicate that in addition to COX2, other mechanisms may also contribute to TRPV1 feed-forward sensitisation, albeit to a seemingly lesser extent than COX2.
Results of the RT-PCR, Western blotting and immunostaining together confirmed the role of COX2 in the capsaicin-induced delayed TRPV1 sensitisation as we detected upregulated synthesis of ptgs2 and COX2. In accordance with findings that a group of primary sensory neurons expresses COX225–28, we found basal ptgs2 and COX2 expression in our neuron samples. Our data indicate that COX2 basal expression, in agreement with previous reports on inducible COX2 expression in a group of primary sensory neurons25,26, was significantly increased after capsaicin exposure. Further, here we report that capsaicin application to cultured primary sensory neurons also results in increased synthesis of COX2 products—though only when cells were presented by the COX2 substrate arachidonic acid. The failure in finding COX2 products with the absence of arachidonic acid in the buffer indicates the concentration of COX2 products without the presence of exogenous arachidonic acid is below the detection threshold when dissociated primary sensory neurons derived from one mouse are divided into control and treated cultures. Interestingly, in addition to several COX products, the concentration of several fatty acids and lipoxygenase products were also increased after arachidonic acid supplementation. Importantly, several of those upregulated lipoxygenase products, for example LTB4 or 15-(S)-HETE, might also contribute to delayed TRPV1 sensitisation; for example, in cells unaffected by the COX and COX2 inhibitors30,31.
Several COX2 products, including PGE2, 15dPGJ2 and PGD2, PGI2, have been implicated in acting on TRPV1-expressing primary sensory neurons13,19,20. Our targeted UPLC-MS study revealed that 4 of the 7 COX products included in the panel we used (PGD2, PGF2a, 15dPGJ2 and 11dTBX2), showed significantly increased synthesis after capsaicin exposure of cultured murine primary sensory neurons. This finding suggests that enzymes downstream of COX2 may exhibit differential expression and/or activation in primary sensory neurons after capsaicin exposure.
Among the upregulated prostaglandins we found PGD2, which previously has been shown to activate PKA type II in TRPV1-expressing primary sensory neurons18. As shown previously, PKA-mediated TRPV1 phosphorylation results in TRPV1 sensitisation15 indicating that PGD2 might indeed sensitise TRPV1. However, Isensee and co-workers18 found prostaglandin D synthase expression in primary sensory neurons that lack TRPV1 activity. Therefore, at present it is not clear whether, in our cultures, TRPV1expressing neurons themselves or non-TRPV1-expressing cells, activated by some agents released from TRPV1-expressing neurons by capsaicin, constitute the source of PGD2.
Another COX-related product we found upregulated, after exposing our cultures to capsaicin, is 15d-PGJ2. This product is synthesised from PGD2 through PGJ232. Previously, 15d-PGJ2 has been reported to activate TRPV1 through covalently binding to the ion channel33.
Interestingly, even though the concentration of PGE2 (the best known, most powerful TRPV1 sensitizer, and widely studied COX product) was increased following capsaicin exposure—this change failed to reach the level of significance. This result might suggest that PGE2 has little role in the development of feed-forward TRPV1 sensitisation. Instead, PGE2 released from monocytes or differentiated macrophages34 may have a greater contribution to a quick sensitisation of TRPV1 during inflammation35.
The novel downstream effects of TRPV1 activation we present here were induced by the exogenous TRPV1 activator capsaicin. These downstream effects can be biologically meaningful only if endogenous TRPV1 activators are also able to induce it. To the best of our knowledge, no data on the TRPV1 activation-evoked TRPV1 sensitisation by endogenous TRPV1 ligands are currently available. However, similarly to capsaicin, endogenous TRPV1 activators also induce Ca2+ influx, which activates ERK1/2 and CaMKII, the signalling molecules that mediate the TRPV1 activation-evoked TRPV1 sensitisation24. Hence, while it must yet be elucidated, it is highly likely that endogenous TRPV1 activators evoke similar COX2-dependent TRPV1 activation-induced TRPV1 sensitisation than capsaicin.
In summary, here we report the phenomenon of feed-forward TRPV1 sensitisation in murine primary sensory neurons, which depends largely on COX2 activity. We also report that COX2 activity is accompanied by upregulated synthesis of the enzyme itself and a significant increase in the synthesis of a number of COX-related products. While COX products released from immunocompetent cells sensitise TRPV1 seconds after acting on TRPV1-expressing primary sensory neurons, agents produced by COX2 in TRPV1-expressing primary sensory neurons themselves appear to prolong the sensitisation state. Hence, the COX2-mediated feed-forward TRPV1 sensitisation represents another ‘layer’ of PG-mediated TRPV1 sensitisation. While the development of this second layer requires ~ 30 min, due to the development of a TRPV1 activation–COX2 upregulation–upregulated PG synthesis–TRPV1 sensitisation–TRPV1 activity reverberating circuit, it may maintain the sensitised state of TRPV1 for a prolonged period of time even when the concentration of PGs are reduced in the interstitial milieu. Interestingly, our data suggest that COX2-dependent sensitisation through peripheral and primary sensory neuron mechanisms may involve different COX-related agents. The relative contribution of TRPV1 sensitisation through immunocompetent and primary sensory neuron mechanisms is not known at present. Further, the receptors involved in the delayed sensitisation process and the signalling pathways have not been elucidated. Nevertheless, these findings, in addition to the “immediate” (within minutes) and “late” (within hours) feed-forward TRPV1 sensitisation (mediated respectively by TRPV1 activators such as anandamide21,22 and secondary gene products such as PARs23 reveal a novel, delayed (~ 30-min) mechanism of feed-forward autocrine signalling leading to TRPV1 sensitisation through the upregulation of immediate early gene and gene products. Therefore, these findings also indicate that, in addition to TRPV1 sensitisation by inflammatory mediators, feed-forward autocrine TRPV1 sensitisation (“autosensitisation”) is multifaceted and that COX2 inhibition in primary sensory neurons is important in reducing inflammatory heat and mechanical hypersensitivity.
Materials and methods
Experiments were conducted in accordance with guidelines published by the International Association for the Study of Pain (IASP)36, UK Animals (Scientific Procedures) Act 1986, revised National Institutes of Health Guide for the Care and Use of Laboratory Animals, Directive 2010/63/EU of the European Parliament and of the Council on the Protection of Animals Used for Scientific Purposes, Good Laboratory Practice and ARRIVE guidelines. A total of 24 wild type C57BL/6 mice were used. Mice were killed humanely following Isoflurane anaesthesia. Experiments were designed and approved by Imperial College London’s Animal Welfare and Ethical Review Body in compliance with Animals (Scientific Procedures) Act1986 (amended in 2012). Every effort was taken to minimize the number of animals.
Culturing primary sensory neurons
Primary sensory neuron cultures were prepared as described37. Briefly, dorsal root ganglia (DRG) from the 3rd cervical to the 6th lumbar segments were harvested, placed into Ham’s Nutrient Mixture F12 supplemented with Ultraser G (2% final concentration), l-glutamine (0.2 mM final concentration), penicillin (100 IU/ml final concentration) and streptomycin (100 μg/ml final concentration) and incubated at 37 °C for 2.5 h with collagenase type IV (312.5–625 IU/ml final concentration) followed by an additional 30 min incubation in the presence of trypsin (2.5 mg/ml final concentration). Ganglia were then washed in F12, triturated then plated onto poly-dl-ornithine-coated coverslips. Cultures were incubated in the supplemented F12 at 37 °C for 24–48 h before being used for further experiments.
Calcium imaging
Imaging was conducted as described previously38. Briefly, cells were loaded with 1 μM FURA-2 (Invitrogen) and 2 mM probenecid (Invitrogen) for 45–60 min in extracellular buffer (NaCl, 150 mM; KCl, 5 mM; CaCl2, 2 mM; MgCl2, 2 mM; glucose, 10 mM and HEPES, 10 mM; pH7.4) at 37 °C. Coverslips were superfused (~ 1.5 ml/min) with the extracellular buffer at 37 °C in a flow chamber. The outlet of the perfusion system was positioned next to the group of cells from which the recordings were obtained. Only one field of view was tested on each coverslip. Cells were exposed to excitation light alternating between the wavelengths of 355 and 380 nM (OptoLED Light Source, Cairn Research, UK). The fluorescent signals emitted in response to each excitation were captured with an optiMOS camera (QImiging, Canada) connected to a PC running the WinFluor software package (John Dempster, Strathclyde University, UK).
Images were analysed offline with the WinFlour and Clampfit (Molecular devices, USA) software packages. Briefly, after establishing the regions of interest, the time course of changes in the fluorescent ratios were examined and exported to Clampfit where the standard deviation (SD) of the baseline activity and the maximum amplitudes of responses were measured. Change in the fluorescent ratio was accepted as a response if the generation of the response corresponded to the drug application and the amplitude of the change was greater than 3 times the SD of the baseline activity. The number of cells in various groups (i.e. sensitizer and nonsensitizer neurons) and the amplitude of responses of cells from at least three independent cultures were pooled and averaged.
Reverse transcriptase polymerase chain reaction (RT-PCR)
RT-PCR was prepared as described previously38. Briefly, DRG cells were washed with Hank’s balanced salt solution (HBSS) buffer (pH 7.4; 138 mM NaCl; 5 mM KCl; 0.3 mM KH2PO4; 4 mM NaHCO3; 2 mM CaCl2; 1 mM MgCl2; 10 mM HEPES; 5.6 mM glucose) and incubated at 37.0ºC in either 500 nM capsaicin solution (capsaicin-treated group) or HBSS (control group) for 5 min, followed by incubation in HBSS for 30 min. RNA was subsequently extracted using the RNeasy Plus Mini Kit (QIAGEN, UK) according to manufacturer’s instructions. RNA was reverse transcribed using Oligo(dT)12–18 primers (500 μg/ml; Invitrogen, UK), dNTP Mix (10 mM; Promega, UK), 5 × First Strand Buffer (Invitrogen, UK) and 0.1 M DTT (Thermo Fisher Scientific, UK) and SuperScript II (Invitrogen, UK).
PCR for COX-2 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), as internal control, were performed using cDNA, 5 × Green GoTaq Reaction Buffer (Promega, UK), 25 mM magnesium chloride, PCR Nucleotide Mix (Promega, UK) and GoTaq Flexi DNA Polymerase (Promega, UK). GAPDH primers were proprietary and supplied by Primerdesign Ltd, UK. Primers for COX-2 (NM_011198) were designed using PrimerBlast and are as follows: forward 5′TCCATTGACCAGAGCAGAGA -3′; reverse: 5′- TGGTCTCCCCAAAGATAGCA-3′. Optimal PCR annealing temperature for COX-2 was determined by gradient PCR annealing at a range from 53.0 to 61.0 °C (not shown). Each amplification of the 30 cycles consisted of 30 s at 95.0 °C, 1 min at 58.0 °C for COX2 or 60.0 °C for GAPDH, and a final 1 min at 72.0 °C. Reactions were performed in thermal cycler Eppendorf Mastercycler Personal (Eppendorf, UK). PCR amplicons along with 100 bp DNA Ladder (New England Biolabs, UK) were visualised on 2% agarose gel using ethidium bromide. Images were captured under UV light using G:Box system (Syngene, UK) and processed with GeneSnap software (Syngene, UK). Digital image analysis of PCR amplicons was conducted using ImageJ software to quantify the intensity of PCR products, determined by the area encompassed by the intensity peaks. Level of COX2 transcription was normalised to the expression level of the housekeeping gene, GAPDH. No modifications of the gel image other than cropping were done. The full gel image is shown in Supplementary Fig. 3.
Western blotting
Cells were incubated in 500 nM capsaicin or in HBSS at 37 °C at 37 °C for 5 min then in HBSS supplemented with 5 μg/mL selective proteasome inhibitor MG-132 in DMSO supplemented for 30 min. 50 μL of NP40 lysis buffer (Invitrogen, UK) supplemented with 1 mM phenyl methane sulfonyl fluoride (PMSF) and 1 × protease inhibitor cocktail (Sigma-Aldrich, USA) was used for protein extraction. The lysates were transferred to Eppendorf tubes and spun down at 14,800 rpm at 4 °C for 10 min and the supernatant collected. The protein concentrations of cell lysates were measured before the SDS-PAGE. The supernatant was mixed with 6 × reducing sample buffer (500 mM Tris pH6.8, 0.1 mg/mL SDS, 34% glycerol, 0.1 mg/mL bromophenol blue, 5% β-mercaptoethanol) and heated at 98 °C for 2 min. An equal mass of proteins was loaded onto 9% polyacrylamide gels. Proteins were separated and transferred onto nitrocellulose membrane (GE Healthcare Life Sciences). The membrane was washed in tris-buffered saline containing 0.1% Tween (Sigma; (TBST) then blocked in 5% non-fat milk or bovine serum albumin (BSA; Sigma) in TBS-T at room temperature for 1 h. The membrane was then probed with 1:2500 diluted anti-COX-2 (ab15191, Abcam) and 1:2000 diluted anti-β-tubulin III (neuronal) (T8578, Sigma-Aldrich), supplemented in 5 mL of 5% non-fat milk or BSA in TBS-T at 4 °C overnight. The reaction was visualised using HRP-conjugated anti-rabbit IgG (7074S, Cell Signalling) and anti-mouse IgG (7076S, Cell Signalling) and the Pierce ECL 2 Western blotting substrate following the manufacturer’s instructions. The membranes were then imaged by the imaging machine GeneGnome XRQ SynGene. Analysis of immunoblots was performed using ImageJ. The intensity of the COX2 signal was normalised to the intensity of the β-tubulin III signal. No modifications on membrane images other than cropping were done. The membrane image from one culture is shown in Supplementary Fig. 4.
Immunostaining
Cells were incubated in 500 nM capsaicin or in HBSS at 37 °C for 5 min, followed by an incubation in HBSS supplemented with 5 μg/mL selective proteasome inhibitor MG-132 in DMSO at 37 °C for 30 min and fixing for 15 min in 4% paraformaldehyde. Cells were permeabilised (0.3% Titon X), blocked in 5% normal goat serum and incubated in anti-COX-2 M19 (sc-1747, Santa Cruz) antibody (1:200) for overnight at 4 °C. The reaction was visualised using an anti-Goat Cross-Adsorbed Alexa Fluor 488 (A-11055, Invitrogen; 1:1000) antibody and covered in Vectashield Hard Set Antifade Mounting Medium with DAPI (H-1500, Vector Laboratories). Images were taken using a Leica DMBL fluorescence microscope (Leica, Germany) with a Retiga 2000R digital camera (QImaging, USA) controlled by Leica Application Suite. All the images of randomly taken fields were taken with the same camera settings and conditions.
Immunofluorescence images without any processing were analysed using ImageJ. The average COX2 and TRPV1 immunofluorescent signals were measured in a group of neurons in randomly selected areas in capsaicin-treated and control coverslips prepared from 3 cultures (> 100 cells/culture). Intensity values from each coverslips were plotted, the threshold for positive immunofluorecent signals and the average signal intensity and the proportion of COX2- and/or TRPV1-expressing cells was established39. No modifications other than cropping were done on images shown in Fig. 2.
Measuring capsaicin-induced eicosanoid production
Cells were incubated at 37 °C for 5 min in capsaicin (500 nM) or HBSS buffer for control. The wells were then incubated for an additional 30 min in HBSS or in HBSS with 10 µM arachidonic acid. Equivolume of 100% methanol was added to wells. Then, cells were scraped, collected in Eppendorf tubes, vortexed and 50µL of the lysate were removed from each sample for protein quantification. The samples were stored at − 20 °C.
Protein quantification was performed using the Pierce BCA protein assay kit (Thermo Fisher Scientific, UK) according to the instructions. Standards and samples were measured in duplicates. Reading was performed at a wavelength of 540 nm in a BioTek ELx800 spectrophotometer (Fisher Scientific, UK) and duplicate readings were averaged.
The rest of the samples were analysed at the MRC-NIHR National Phenome Centre (UK) using a novel targeted assay developed for the quantification of 48 lipid mediators of inflammation40. Briefly, separation was performed using a Waters HSS T3 column. Samples were then analysed in negative ion mode in a Waters Xevo TQ-S triple quadrupole mass spectrometer (MS) with an electrospray ionisation (ESI) source. Concentrations detected by UPLC-MS were first normalised to the protein content of the samples. Then, normalised compound concentrations of the capsaicin-treated cells were normalised to values established in the respective control.
Statistical analysis
Normal distribution of data was assessed using the Shapiro–Wilk test. Significant change in the number of sensitising/non-sensitising and immunostained cells was assessed using Fischer’s exact test. Amplitudes in Ca2+ imaging were compared using Student’s t-test or ANOVA followed by Tukeys post-hoc test as appropriate. Changes in both expression values and the concentration of lipid mediators were compared using Student’s t-tests. In the case of the concentration of lipid mediators, Student’s test values were corrected for false discovery rate using the Benjamini–Hochberg approach. Values are expressed as average ± standard error of mean (SEM). Differences were regarded as significant at p/q < 0.05. p values above 0.0001 are given as exact values, whereas below 0.0001, they are given as p < 0.0001.
Supplementary Information
Acknowledgements
JdSV and PLM have been supported, respectively by a PhD studentship from Fundacao para a Ciencia e a Tecnologia (Portugal) and a British Journal of Anaesthesia Research Grant. The authors are grateful for Ms Berenike Buhl for her technical assistance.
Author contributions
T.L.: calcium imaging. G.W.: Western-blotting, immunostaining, writing up. V.C.C.H.: RT-PCR, writing up. D.S.: UPLC-MS, writing up. J.dS.V.: writing up. P.L.M.: western-blotting, immunostaining. I.N.: conceptualisation, managing/leading project, writing up.
Data availability
This study includes no data deposited in external repositories.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-82829-6.
References
- 1.Placek K, Schultze JL, Aschenbrenner AC. Epigenetic reprogramming of immune cells in injury, repair, and resolution. J. Clin. Invest. 2019;129:2994–3005. doi: 10.1172/JCI124619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berta T, Qadri Y, Tan PH, Ji RR. Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain. Expert Opin. Ther. Targets. 2017;21:695–703. doi: 10.1080/14728222.2017.1328057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagy, I. Sensory processing: primary afferent neurons/DRG. Anesthetic Pharmacology: Physiologic Principles and Clinical Practice, eds: Evers and Maze, 187–197 (2004).
- 4.Yekkirala AS, Roberson DP, Bean BP, Woolf CJ. Breaking barriers to novel analgesic drug development. Nat. Rev. Drug Discov. 2017;16:545–564. doi: 10.1038/nrd.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 6.Caterina MJ, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 7.Woolf CJ, Ma Q. Nociceptors–noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Amadesi S, et al. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J. Physiol. 2006;575:555–571. doi: 10.1113/jphysiol.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukuoka T, et al. VR1, but not P2X(3), increases in the spared L4 DRG in rats with L5 spinal nerve ligation. Pain. 2002;99:111–120. doi: 10.1016/s0304-3959(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 10.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 11.11Kao, D. J. et al. CC chemokine ligand 2 upregulates the current density and expression of TRPV1 channels and Nav1.8 sodium channels in dorsal root ganglion neurons. J. Neuroinflamm.9, 189. 10.1186/1742-2094-9-189 (2012). [DOI] [PMC free article] [PubMed]
- 12.Khan AA, et al. Tumor necrosis factor alpha enhances the sensitivity of rat trigeminal neurons to capsaicin. Neuroscience. 2008;155:503–509. doi: 10.1016/j.neuroscience.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 13.Moriyama T, et al. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol. Pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- 15.Rathee PK, et al. PKA/AKAP/VR-1 module: A common link of Gs-mediated signaling to thermal hyperalgesia. J. Neurosci. 2002;22:4740–4745. doi: 10.1523/JNEUROSCI.22-11-04740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Buren JJ, Bhat S, Rotello R, Pauza ME, Premkumar LS. Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain. 2005;1:17. doi: 10.1186/1744-8069-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isensee J, et al. Subgroup-elimination transcriptomics identifies signaling proteins that define subclasses of TRPV1-positive neurons and a novel paracrine circuit. PLoS ONE. 2014;9:e115731. doi: 10.1371/journal.pone.0115731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience. 1989;32:577–580. doi: 10.1016/0306-4522(89)90280-7. [DOI] [PubMed] [Google Scholar]
- 20.Taiwo YO, Levine JD. Effects of cyclooxygenase products of arachidonic acid metabolism on cutaneous nociceptive threshold in the rat. Brain Res. 1990;537:372–374. doi: 10.1016/0006-8993(90)90389-s. [DOI] [PubMed] [Google Scholar]
- 21.Nagy I, et al. NAPE-PLD is involved in anandamide synthesis in capsaicin-sensitive primary sensory neurons. J. Physiol. Sci. 2009;59:422–422. [Google Scholar]
- 22.van der Stelt, M. et al. Anandamide acts as an intracellular messenger amplifying Ca2+ influx via TRPV1 channels. EMBO J.24, 3026–3037. 10.1038/sj.emboj.7600784 (2005). [DOI] [PMC free article] [PubMed]
- 23.Chen D, Wang Z, Zhang Z, Zhang R, Yu L. Capsaicin up-regulates protease-activated receptor-4 mRNA and protein in primary cultured dorsal root ganglion neurons. Cell. Mol. Neurobiol. 2013;33:337–346. doi: 10.1007/s10571-012-9899-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Daugherty SL, de Groat WC. Activation of CaMKII and ERK1/2 contributes to the time-dependent potentiation of Ca2+ response elicited by repeated application of capsaicin in rat DRG neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300:R644–654. doi: 10.1152/ajpregu.00672.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Kim JS, van Wijnen AJ, Im HJ. Osteoarthritic tissues modulate functional properties of sensory neurons associated with symptomatic OA pain. Mol. Biol. Rep. 2011;38:5335–5339. doi: 10.1007/s11033-011-0684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fehrenbacher JC, Burkey TH, Nicol GD, Vasko MR. Tumor necrosis factor alpha and interleukin-1beta stimulate the expression of cyclooxygenase II but do not alter prostaglandin E2 receptor mRNA levels in cultured dorsal root ganglia cells. Pain. 2005;113:113–122. doi: 10.1016/j.pain.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 27.Samad TA, et al. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 28.Araldi D, et al. Peripheral inflammatory hyperalgesia depends on the COX increase in the dorsal root ganglion. Proc. Natl. Acad. Sci. USA. 2013;110:3603–3608. doi: 10.1073/pnas.1220668110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagy I, Friston D, Valente JS, Perez JVT, Andreou A. P. Prog. Drug Res. 2014;68:39–76. doi: 10.1007/978-3-0348-0828-6_2. [DOI] [PubMed] [Google Scholar]
- 30.Koskela H, Purokivi M, Nieminen R, Moilanen E. The cough receptor TRPV1 agonists 15(S)-HETE and LTB4 in the cough response to hypertonicity. Inflamm. Allergy Drug Targets. 2012;11:102–108. doi: 10.2174/187152812800392814. [DOI] [PubMed] [Google Scholar]
- 31.Zinn S, et al. The leukotriene B4 receptors BLT1 and BLT2 form an antagonistic sensitizing system in peripheral sensory neurons. J. Biol. Chem. 2017;292:6123–6134. doi: 10.1074/jbc.M116.769125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 33.Shibata T, et al. Identification of a prostaglandin D2 metabolite as a neuritogenesis enhancer targeting the TRPV1 ion channel. Sci. Rep. 2016;6:21261. doi: 10.1038/srep21261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sha W, Brune B, Weigert A. The multi-faceted roles of prostaglandin E2 in cancer-infiltrating mononuclear phagocyte biology. Immunobiology. 2012;217:1225–1232. doi: 10.1016/j.imbio.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Grosch S, Niederberger E, Geisslinger G. Investigational drugs targeting the prostaglandin E2 signaling pathway for the treatment of inflammatory pain. Expert Opin. Investig. Drugs. 2017;26:51–61. doi: 10.1080/13543784.2017.1260544. [DOI] [PubMed] [Google Scholar]
- 36.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 37.Nagy I, Rang HP. Similarities and differences between the responses of rat sensory neurons to noxious heat and capsaicin. J. Neurosci. 1999;19:10647–10655. doi: 10.1523/jneurosci.19-24-10647.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varga A, et al. Anandamide produced by Ca(2+)-insensitive enzymes induces excitation in primary sensory neurons. Pflugers Arch. 2014;466:1421–1435. doi: 10.1007/s00424-013-1360-7. [DOI] [PubMed] [Google Scholar]
- 39.Sousa-Valente J, et al. Inflammation of peripheral tissues and injury to peripheral nerves induce differing effects in the expression of the calcium-sensitive N-arachydonoylethanolamine-synthesizing enzyme and related molecules in rat primary sensory neurons. J. Comp. Neurol. 2017;525:1778–1796. doi: 10.1002/cne.24154. [DOI] [PubMed] [Google Scholar]
- 40.Wolfer AM, Gaudin M, Taylor-Robinson SD, Holmes E, Nicholson JK. Development and validation of a high-throughput ultrahigh-performance liquid chromatography-mass spectrometry approach for screening of oxylipins and their precursors. Anal. Chem. 2015;87:11721–11731. doi: 10.1021/acs.analchem.5b02794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study includes no data deposited in external repositories.