Fig. 4. Membrane trafficking of αS at the synaptic termini.
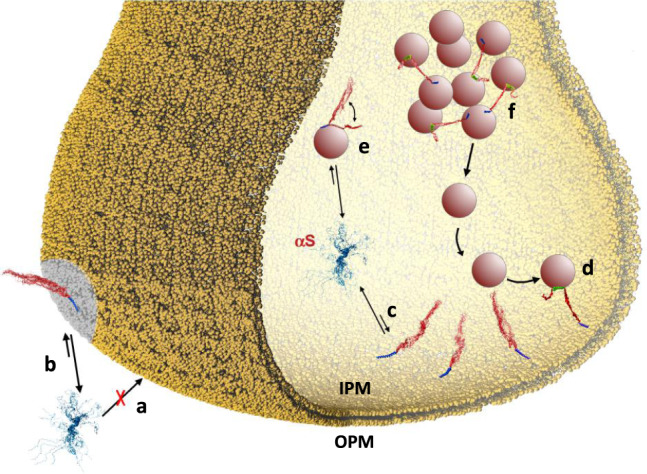
Schematic illustration of the different interactions between αS and biological membranes at the synaptic termini. a αS binds to OPM with negligible affinity. b When the content of GMs in the membrane composition increases, such as in the case of some neurodegenerative disorders52 and in lipid rafts54,65, the affinity of αS for OPM is considerably enhanced. c The binding affinity of αS for IPM is significantly higher than that observed in the case of OPM. Upon interaction with IPM, αS adopts a conformation where only the N-terminal anchor (blue) is tightly bound to the membrane, with the region of residues 65–140 (red) having negligible association with the membrane surface. d This peculiar conformation has significant propensity to promote a double-anchor mechanism (N-terminal anchor in blue; second anchor spanning residues 65–97 in green) that stabilises the SV docking onto the IPM surface in an αS concentration-dependent manner. The IPM binding by αS competes with the binding to SVs (e), which is involved in the mainteinance of pools of vesicles (f) from which SVs diffuse toward the active zone.
