Abstract
The autonomic nervous system (ANS), which consists of antagonistic sympathetic (adrenergic) and parasympathetic (cholinergic) arms, has emerged as an important regulator of neoplastic development, yet little is known about its role in multiple myeloma (MM). Clinical findings that anti-adrenergic β-blocker intake reduces risk of disease-specific death and overall mortality in patients with MM have indicated that adrenergic input may worsen myeloma outcome. However, preclinical studies using β-adrenergic receptor agonists or antagonists produced controversial results as to whether sympathetic pathways promote or inhibit myeloma. Retrospective outcome data demonstrating that high message levels of cholinergic receptor genes predict inferior survival in the Multiple Myeloma Research Foundation CoMMpass trial suggest that parasympathetic input may drive myeloma progression in a subset of patients. Here we review the ill-defined role of the ANS in MM, put myeloma in the context of other cancers, and discuss knowledge gaps that may afford exciting research opportunities going forward.
Keywords: Plasma cell malignancy, Sympathetic and parasympathetic tone, Adrenergic and cholinergic signaling
1. Introduction
Multiple myeloma (MM) is a neoplasm of terminally differentiated, immunoglobulin-producing B-lymphocytes, called plasma cells, that depend on the bone marrow microenvironment (BMM) for growth and survival. Quintessential disease manifestations include serum paraprotein, focal bone loss, hypercalcemia and kidney damage. With an estimated 30 thousand cases annually, MM is the second most common blood cancer in the United States. Frank myeloma is preceded by the premalignant condition, monoclonal gammopathy of undetermined significance (MGUS) [1]. Owing to both newly developed myeloma drugs and the refinement of therapeutic regimens that combine high-dose chemotherapy (melphalan) with hematopoietic stem cell (HSC) transplantation, the outcome of MM has significantly improved in recent years [2]. Nonetheless, after a period of successful therapy, the great majority of patients relapse with drug-resistant aggressive disease that leaves few if any therapeutic options. Recent progress in our understanding of the mechanism with which the BMM supports myeloma has been expertly reviewed [3]. Strategies for targeting the BMM to block the MGUS-to-MM transition and thus prevent frank myeloma from manifesting itself are also emerging [4]. In contrast, little attention has been paid to an integral yet understudied player in the BMM: the autonomic nervous system (ANS). Autonomic nerves, which can be divided into an adrenergic “fight-or-flight” sympathetic branch and a cholinergic “rest-and-digest” parasympathetic branch, infiltrate the BMM and interact with resident cells presumably including myeloma cells (Fig. 1). In the past the ANS has been perceived as a passive bystander in myeloma, yet recent research reviewed in the following has implicated autonomic nervous input in the pathophysiology and outcome of myeloma. Here, we summarize the new findings, put myeloma in the context of other blood and solid cancers, and discuss knowledge gaps that may afford exciting research opportunities going forward.
Fig. 1.
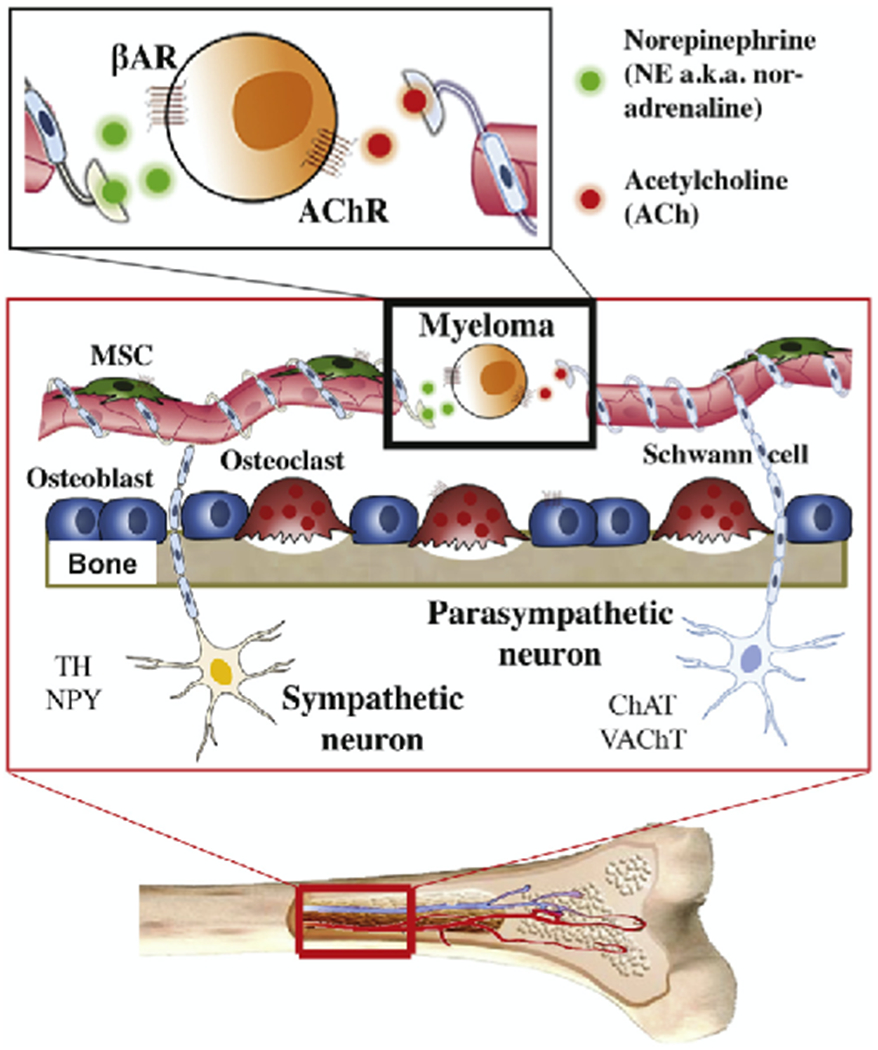
Hypothetical scheme of ANS control of myeloma in the BMM. BM in highly innervated by sympathetic and parasympathetic nerve fibers, the topographical distribution of which can be visualized with the help of immunostaining for neuronal markers. Tyrosine hydroxylase (TH), the rate-limiting enzyme of catecholamine synthesis, identifies sympathetic nerves, which are typically warped in a spiral pattern around blood vessels in the BM parenchyma and periosteum [81,82]. Neuropeptide Y (NPY), a neurotransmitter stored at sympathetic nerve endings, reveals a distribution pattern similar to that of TH fibers, yet NPY fibers are often smaller and less abundant [82]. Choline acetyltransferase, ChAT, the key enzyme for acetylcholine production by cholinergic neurons, detects parasympathetic nerves, which are observed in proximity to hematopoietic islets under normal conditions [83]. Vesicular acetylcholine transporter (VAChT), a member of the vesicular amine transporter family, visualizes parasympathetic fibers in intertrabecular spaces [84]. Just like bone cells and mesenchymal stem (MSC) and other cells residing in the hematopoietic BM, myeloma cells are exposed to and regulated by adrenergic (sympathetic) and cholinergic (parasympathetic) signals. The former are transmitted by norepinephrine (NE), which binds to β-adrenergic receptors (β-AR). The latter are transmitted by acetylcholine, which binds to cholinergic receptors (AChR). The role of adrenergic/cholinergic signaling in myeloma development and progression is ill understood.
2. Pharmacological inhibition of sympathetic input improves myeloma outcome
A recent retrospective outcome analysis of multiple myeloma demonstrated that anti-adrenergic β-blocker intake led to reduced risk of disease-specific death and overall mortality compared to non-β-blocker cardiac drug use or no use of cardiac drugs [5] (Table 1). The result, which indicated that dampening adrenergic signaling benefits patients with myeloma (Fig. 2A), agreed with preclinical studies using laboratory mice (Fig. 2B) and findings from a recent clinical trial on the broad-spectrum β-blocker, propranolol, [6] demonstrating that adrenergic inhibition down regulates the conserved transcriptional response to adversity (CTRA) in myeloma [7]. The result was also consistent with population-based studies associating β-blocker intake with reduces mortality in other cancers [8–10] and a large body of evidence that sympathetic activation due to psychological distress including anxiety and depression results in heightened mortality in cancer [11]. In a meta-analysis of myeloma survival, psychological distress was associated with significantly inferior death outcome at a hazard ratio of 2.36 [12]. A prospective study, which arrived at the same conclusion, demonstrated that myeloma and lymphoma patients with depressive symptoms have an approximately twofold risk elevation for all-cause mortality [13]. Due in part to intense treatment regimens requiring long periods of hospitalization [14], chronic stress and depression are prevalent in patients with myeloma and other hematological cancers [15]. In a study on myeloid neoplasia, for example, 40% and 31% of patients met National Comprehensive Cancer Network (NCCN) and Hospital Anxiety and Depression Scale (HADS) criteria for distress and anxiety, respectively; and one of eight patients (12.5%) was diagnosed with outright depression [16]. Clinical trials are warranted to determine whether myeloma patients may benefit from interventions to enhance positive psychological resources using psychotherapy [17,18] and antidepressants [19]. Simple yet efficient tools for assessing mental health and psychological burden are available to support trials of this sort [20], and to develop viable strategies for enhanced quality of life (QOL) and improved outcome of patients with myeloma.
Table 1.
Evidence for impact of β-adrenergic signaling in patients with myeloma (rows 1–5) or continuous human myeloma cell lines (rows 6–10).
| Type of investigation | Main finding or clinical question | Year published | Ref. |
|---|---|---|---|
| Retrospective outcome analysis | In patients with multiple myeloma, β-blocker intake is associated with reduced disease-specific death and overall mortality | 2017 | 5 |
| Meta-analysis of prospective cohort studies | Psychological distress (depression, anxiety) results in 2-fold increase in disease-specific mortality | 2017 | 12 |
| Clinical pilot study (NCT01899326) | Stimulation of adrenergic activity by tricyclic antidepressant desipramine enhances HSC a mobilization induced by G-CSFb | 2017 | 54 |
| Clinical pilot study (NCT02420223) | Do patients with myeloma that undergo HSC transplantation benefit from anti-adrenergic treatment with propranolol? | 2018 | 6 |
| Phase 2 biomarker trial (NCT02420223) | In patients with myeloma undergoing HSC transplantation, treatment using unspecific β-blocker propranolol inhibits conserved transcriptional response to adversity (CTRA) | 2020 | 7 |
| Preclinical in vitro study using one HMCLc | Norepinephrine (noradrenaline) stimulates interleukin 6 (IL-6) dependent FLAM-76 myeloma cells | 2008 | 23 |
| High-throughput drug interaction study in vitro | β2ARd agonists synergize with backbone myeloma drugs in myeloma cell killing | 2012 | 24 |
| Preclinical in vitro study using one HMCL | Propanol inhibits growth and promotes apoptosis of U266 myeloma cells | 2013 | 21 |
| Preclinical in vitro study using three HMCLs | β1ARe agonist dobutamine inhibits myeloma in a MAPK f dependent manner | 2016 | 25 |
| Preclinical in vitro study using one HMCL | Epinephrine (adrenaline) enhances growth, proliferation and chemoresistance of U266 myeloma cells | 2017 | 22 |
Hematopoietic stem cell.
Granulocyte-colony stimulating factor.
Human myeloma cell line.
β2 adrenergic receptor.
β1 adrenergic receptor.
Mitogen-activated protein kinase.
Fig. 2.
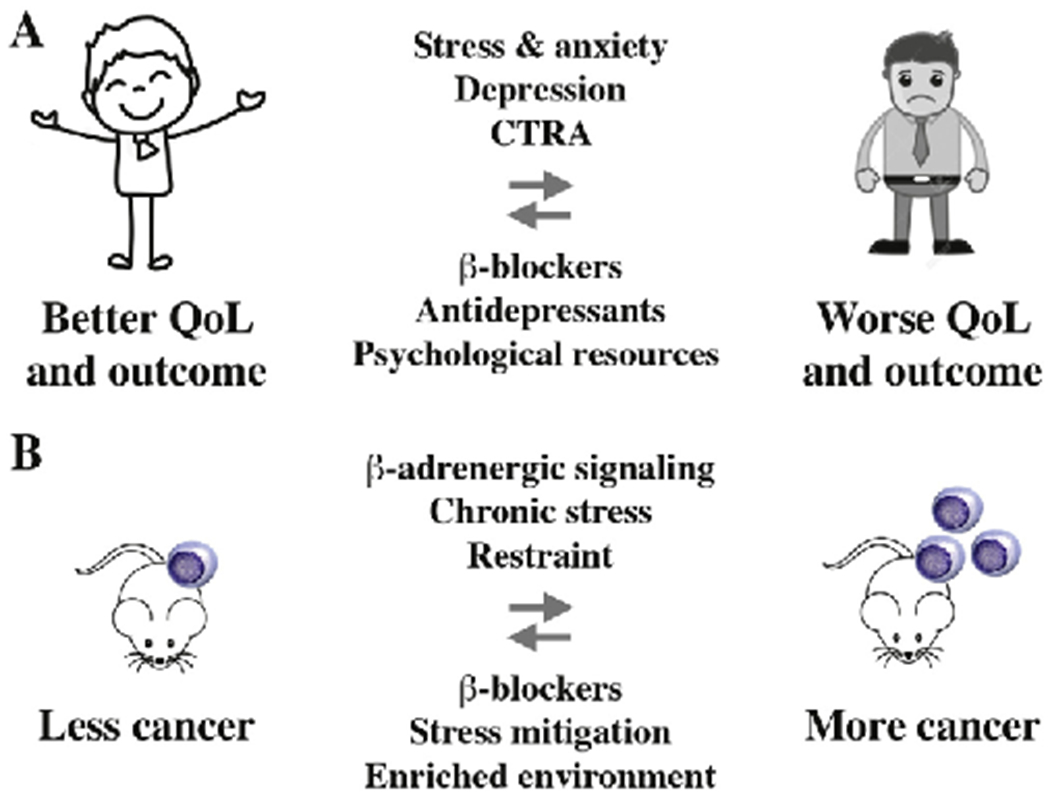
Dampening adrenergic signaling benefits patients with myeloma. Panel A shows that β-blocker intake, which dampens adrenergic signaling, reduces disease-specific mortality in patients with myeloma (arrow pointing left). Conversely, psychological distress (anxiety, depression), which leads to increased adrenergic signaling, is associated with inferior survival and reduced quality of life (QoL; arrow pointing right). Stress and anxiety relief using antidepressants and/or psychotherapy may improve outcome of myeloma by virtue of a mechanism that involves adrenergic regulation of immune cells in the BMM [85]. Dysregulation of adrenergic and other stress-related signaling pathways is evident at the transcriptomic level, as recently shown in a study on lethal prostate cancer [86] and, more broadly, in the context of a newly developed psycho-oncological framework [87] termed conserved transcriptional response to adversity (CTRA) [88]. In accordance with that, the broad-spectrum β-blocker propranolol [6] inhibited the CTRA in a recent clinical trial in myeloma [7]. Panel B depicts the principal outcome of preclinical studies using laboratory mice that linked chronic stress (elevated adrenergic signaling) and cancer (right-pointing arrow) [29,60,89–92]. In contrast, mice housed in a stress-mitigated, enriched environment or treated with anti-adrenergic β-blocker exhibited reduced rates of malignant growth, such as melanoma and colon cancer (left-pointing arrow). Tumor inhibition relied on a pathway that involved downregulation of leptin production in adipocytes in response to β-adrenergic input [93].
3. Divergent impact of sympathetic input modulation on myeloma growth and survival
Consistent with the clinical and epidemiologic evidence described above, preclinical studies have demonstrated that the β-blocker propranolol hampers proliferation and survival of the myeloma cell line U266 [21]. Conversely, adrenergic receptor stimulation with epinephrine (adrenaline) promoted growth and chemoresistance of these cells [22], and norepinephrine (noradrenaline) stimulated the growth of IL-6 dependent FLAM-76 myeloma cells [23] (Table 1, rows 6, 8 and 10). Although these findings support the notion that blocking β-adrenergic signaling may provide a new myeloma treatment approach, other preclinical studies suggest the opposite. Increased rather than decreased β-adrenergic signaling killed myeloma in a drug interaction assay that demonstrated synergism of the β2 adrenergic receptor (β2AR) agonist, salmeterol, with backbone myeloma drugs [24]. Similarly, another β2AR agonist, dobutamine, killed myeloma in cell culture via down regulation of MAPK signaling [25] (Table 1, rows 7 and 9). Taken together, the in vitro results are inconsistent and suggest that the beneficial effect of adrenergic inhibition on myeloma outcome in patients can perhaps better be explained by invoking an indirect mechanism, such as the impact of sympathetic signaling on the immune system or BMM. Current views on adrenergic control of adaptive immune responses [26] and the role of autonomic BM innervation in normal immune function [27] are compatible with the hypothesis that pharmacological inhibition of sympathetic input bolsters anti-myeloma immune responses. In support of that possibility, β-blocker usage improved the survival of patients with metastatic melanoma undergoing immunotherapy [28] and chronic stress in patients with breast and colon cancer was found to dampen anti-tumor immunity by virtue of a pathway that included M2 macrophage polarization [29] and a shift from the Th1 to the Th2 response [30]. Preclinical results on enhanced tumor-suppressive cytotoxic T-cell responses in both β2AR-deficient mice [31] and normal mice treated with β-blockers [28] are also in line with the possibility that adrenergic inhibition boosts the immune response to cancer. Dedicated clinical trials on immune regulatory effects of β-blockers in patients with myeloma and the potential utility of these drugs to enhance immune therapies of myeloma are warranted.
4. Autonomic control of skeletal homeostasis may impact myeloma bone disease
Because bone remodeling is modulated by ANS activity under normal and pathological conditions, it seems reasonable to postulate that the general and focal bone loss seen in patients with myeloma is also impacted by autonomic signals. ANS regulates skeletal homeostasis by means of an “autonomic tone;” i.e., the net result of the sympathetic and parasympathetic input that promotes bone resorption and bone formation, respectively [32]. In sync with that, anti-adrenergic β-blockers have beneficial effects on rebuilding bone mineral density and reducing fracture risk [33]. The full impact of autonomic signals on myeloma bone disease (MBD) has not yet been determined, but circumstantial evidence reviewed by Olechnowicz and Edwards [34] suggests that the sympathetic nervous system (SNS) plays a significant role. Accordingly, psychological stress and anxiety experienced by patients with myeloma may cause SNS-dependent bone loss, using a mechanism that relies on the β-adrenergic pathway in osteoblasts to increase the effect of signals that inhibit osteoblast but activate osteoclast function (Fig. 3A) [34]. An important treatment-related connection of MBD with autonomic nerve damage and adrenergic signal strength is bortezomib (proteasome inhibitor) induced peripheral neuropathy [35,36]. The underlying pathophysiology is poorly understood but appears to involve NFκB-dependent downregulation of brain-derived neurotrophic factor (BDNF) [36–38], a newly emerged serum marker for risk assessment of peripheral neuropathy in patients with myeloma [39]. Because bortezomib exerts welcome anabolic effects on bone, research is under way to deliver the drug to target sites in bone without increasing the risk of neuropathy [40]. In sum, elucidating the impact and mechanism with which the ANS modulates MBD is an interesting area for future research.
Fig. 3.
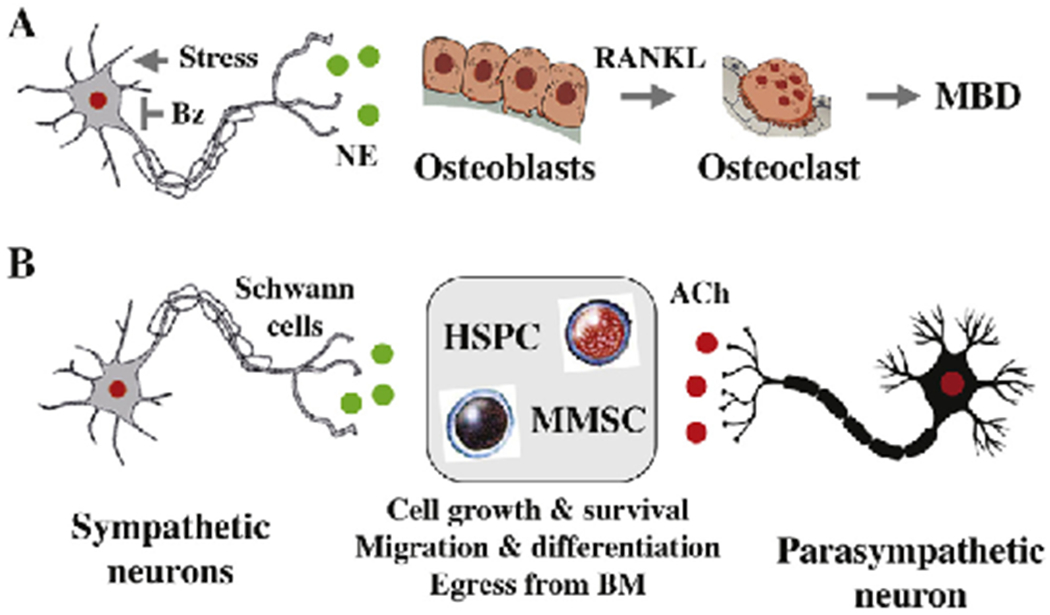
Proposed mechanism of ANS control of myeloma bone disease and sternness. Evidence that autonomic innervation of bone and bone marrow is crucial for skeletal [32] and hematopoietic homeostasis [94,95] suggests that the ANS is also involved in myeloma bone disease (MBD) and myeloma sternness. Autonomic nerve fibers form synapse-like structures with bone cells such as osteoblasts and osteoclasts (panel A) as well as hematopoietic stem and progenitor cells (HSPC, panel B). Neurotransmitter NE and ACh release at the synapse stimulates autonomic receptors on target cells, initiating downstream signal transduction. It is possible that high sympathetic tone caused by chronic stress promotes MBD through increased osteoblast secretion of RANKL, leading to enhanced osteoclast-dependent bone resorption (panel A) [95–97]. It is also possible but has not been shown that the well-established anabolic effect of bortezomib (Bz) on bone relies, in part, on Bz-induced sympathetic neuropathy that curbs adrenergic signaling (panel A). Whether multiple myeloma stem cell (MMSC) function is governed by autonomic signals, similar to HSC and MSC function, is a task for future research (panel B). Encouragement for taking on this challenge is provided by reports that other types of stem cell-like cancer cells (breast, prostate) are regulated by autonomic signals. See main text for details.
5. Autonomic control of hematopoietic stem cell activity may extend to myeloma stem cells
Biological pathways that govern ANS control of normal hematopoietic stem cell (HSC) activity [41] are of interest to myeloma because they may also govern dormancy and activation of multiple myeloma stem cells (MMSCs), enigmatic cancer stem cell-like cells that are of great relevance for tumor relapse and acquisition of drug resistance in myeloma [42]. Similar to the regulation of skeletal homeostasis discussed above, the sympathetic input to hematopoietic sternness is better defined than its parasympathetic counterpart. Sympathetic nerves are an intrinsic constituent of the HSC niche and participate in both niche-driven blood cancers and niche remodeling by cancer cells [43]. Sympathetic signaling is also involved in early niche development [44] and, conversely, age-dependent niche deterioration brought about by adrenergic nerve degeneration [45]. The latter has been modeled in mice, in which surgical denervation or genetic ablation of β3AR led to premature HSC aging [46]. It is unclear whether these results can be extrapolated to MMSCs, but recent findings on adrenergic support of breast cancer stem cells [47] and sympathetic signal-dependent re-activation of quiescent, BM-resident prostate cancer cells [48] point to a broader relevance across the cancer spectrum. Of note, hyperactivation of adrenergic signaling may be as detrimental for stem cell function as loss of signaling. This has been recently shown for chronic stress-induced greying of black laboratory mice that could be attributed to the SNS hyperactivation-dependent depletion of melanocyte stem cells in hair follicles [49]. Whether cancer stem cell-like cells exhibit similar susceptibilities is not known. A schematic overview of ANS control of HSC function, with a MMSC and a mesenchymal stem cell (MSC) shown in a neighboring niche, is presented in Fig. 3B. Defining what role, if any, sympathetic nerves may play in creating and maintaining MMSC survival and proliferation niches is an important task for future research.
6. Autonomic control of stem cell mobilization in myeloma treatment
Sympathetic signaling is also involved in induced mobilization of HSCs [43], an important aspect of myeloma treatment protocols that involve autologous or allogeneic bone marrow transplantation (BMT). Sympathetic input governs, in part, egress of stem cells from bone marrow niches into the peripheral blood stream [50]. This relies on a molecular pathway that includes adrenergic activation of the β3 receptor on BM stromal cells and reduced expression of chemokine receptor ligand CXCL12 (C-X-C motif chemokine ligand 12) [51]. The ligand binds to CXCR4 (C-X-C motif chemokine receptor 4) on HSCs and malignant plasma cells, providing not only a crucial mechanism for the retention of these cells in the bone marrow but also a promising opportunity for therapeutic targeting [52,53]. Stimulation of SNS neurons with granulocyte-colony stimulating factor (G-CSF) potentiates the sympathetic tone by increasing norepinephrine availability due to reuptake inhibition [54]. Desipramine, a FDA-approved tricyclic antidepressant, takes advantage of this mechanism to increase sympathetic activity and, thereby, synergize with G-CSF in HSC mobilization in mice [54] and patients with myeloma [55]. Indeed, an open-label single-arm pilot study on autologous stem cell transplantation in myeloma showed that the combination of desipramine and G-CSF is safe and, importantly, results in improved HSC mobilization versus G-CSF alone [55]. This backdrop demonstrates that elucidating ANS pathways of hematopoietic stem and progenitor mobilization may refine established BMT protocols and render them more effective. What is more, research along this line may lead to new strategies for flushing out quiescent myeloma cells, including stem cell-like cells, from their survival-protecting BM niche to the peripheral circulation. This may force them to re-enter the active cell cycle and thus become vulnerable to killing using conventional cytostatic agents.
7. Widespread sympathetic control of cancer progression suggests complicity in myeloma
The involvement of the ANS in the natural history of solid and hematologic cancers is increasingly recognized [56,57]; however, dedicated studies on myeloma are lacking. While both arms of autonomic tissue control have been firmly implicated in solid cancer development (see the next section for a brief discussion of cholinergic pathways), the bulk of evidence for blood cancers points to the sympathetic arm. Thus, in an orthotropic mouse model of acute lymphoblastic leukemia (ALL), two weeks of daily restraint stress (accompanied by elevated sympathetic tone) enhanced tumor progression, whereas treatment of mice using the β-blocker propranolol slowed it down [58]. This model of ALL provides an example of SNS-promoted oncogenesis by virtue of BMM remodeling [59]. The elucidation of the mechanism with which sympathetic input drives cancer began with pioneering studies on mouse models of human cancer [29,60,61]. For example Magnon et al. analyzed sympathetic innervation of prostate cancer in mice, demonstrating that sympathetic denervation – either by means of surgical cutting or injection of neurotoxic drugs – suppresses tumor progression and metastasis [61]. The most well-understood input is the β2-adrenergic signal delivered by sympathetic nerves to cancer and bystander cells equipped with β2AR. Binding of (nor)epinephrine to the receptor activates the c-AMP/PKA pathway that regulates many aspects of cancer biology [62,63]. Additional mechanisms of β2AR-dependent tumor promotion include AKT-dependent metabolic reprogramming and inhibition of autophagy, as seen in hepatocellular carcinoma [64]; neoangiogenesis and remodeling of the tumor microenvironment, observed in ovarian [60] and prostate cancer [65]; and neurotrophin-induced outgrowth of nerve ends (axogenesis) found in pancreatic ductal adenocarcinoma [66]. Elevated axogenesis resulting in increased overall tumor innervation may also be accomplished by tumor cell-released exosomes [67].
In contrast to the results summarized above, two landmark studies on myeloid malignancy progression in mice have demonstrated that sympathetic input can also inhibit neoplastic growth. In this case, sympathetic neuropathy (diminished adrenergic signaling) promoted myeloid tumor development, whereas increased adrenergic signaling (upon treatment of mice using β2 or β3 agonists) protected sympathetic nerves and suppressed malignant growth [68,69]. The neuropathy seen in this model system resulted from proinflammatory factors secreted by tumor cells in the BMM. This is the stark opposite of the neuroprotective and axon-nurturing effect of pancreatic cancer cells mentioned above. Taken together, the findings indicate that – depending on type of malignancy and specific features of the model system employed – sympathetic pathways may promote or inhibit blood cancer development. This raises an urgent need for clarifying how SNS control plays out in the natural history of plasma cell neoplasia including MM. Mechanistic studies of this kind are difficult to pursue in humans but can be readily carried out with the assistance of a genetically engineered mouse model (GEMM) of human myeloma available in our laboratory (Fig. 4). This model takes advantage of adoptive transfer of oncogene-activated premalignant B cells genetically “hard wired” to undergo neoplastic plasma cell development in a preconditioned host. Among the many strengths of the model is the opportunity to compare the impact of a potential driver of oncogenesis, such as adrenergic signaling, in tumor precursors vs bystander cells in the tumor microenvironment (TME).
Fig. 4.
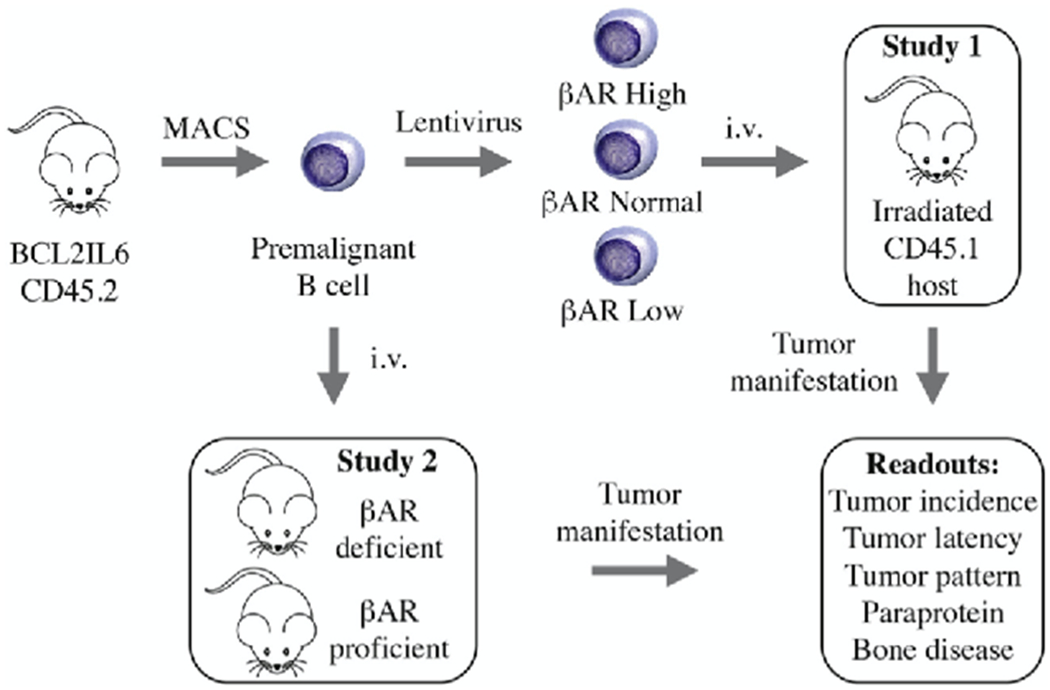
Experimental strategy to evaluate the role of adrenergic signaling in plasma cell tumor (PCT) development in mice. Adoptive B-cell transfer provides a flexible platform for assessing role and significance of adrenergic input in the natural history of myeloma-like PCTs. Effects on tumor precursors (Study 1) can be distinguished from those on the TME (Study 2), as recently shown in a study on the impact of IL-6 (interleukin 6) on PCT development [98]. Briefly, donor B cells are isolated from double-transgenic BCL2IL6 mice on the genetic background of BALB/c, using MACS® magnetic bead columns (cartoon to the upper left). Next, B cells are genetically modified in short-term cell culture using lentiviral gene transduction, which results in enforced expression of a βAR gene of interest (βAR High) or RNAi-mediated downregulation of βAR expression (βAR Low). Transfection with non-coding “empty” virus that leaves endogenous βAR expression unchanged is used as control (βAR Normal). Adoptive transfer of transfected B cells to sub-lethally irradiated hosts congenic for CD45.1 generates 3 cohorts of mice distinguished only by the level of βAR message in tumor precursors (upper right). Tumor incidence, latency and pattern will serve as endpoints of the study. Specific antibodies for the two CD45 allotypes involved provide a convenient tool for monitoring engraftment and neoplastic expansion of donor cells in host tissues, using flow cytometry and immunohistochemistry as measurement tools. Transfer tumor precursors to host mice that are either deficient or proficient in βAR signaling (Study 2) may show whether βAR signaling in the TME is critical for PCT development.
8. Possible role of cholinergic signaling in myeloma progression and outcome
Clear-cut experimental evidence of parasympathetic input contributions to tumor progression is currently limited to solid cancers, in which acetylcholine may be produced by two principal sources: the parasympathetic nerve in the TME and the tumor cell itself. Prostate cancer cells, for example, co-express the key enzyme for acetylcholine synthesis, ChAT (choline acetyltransferase), and one of the receptors acetylcholine is binding to, CHRM3 (cholinergic muscarinic receptor 3). The ability of the tumor cells to secrete acetylcholine may result in high local concentrations of the neurotransmitter and, thereby, enable an autocrine cholinergic loop that drives tumor progression. Consistent with that, overexpression of CHRM3, or receptor activation using carbachol, promoted prostate cancer growth and castration resistance in mice [70], whereas treatment of mice with the selective CHRM3 antagonist, darfenacin, inhibited these phenotypes [70]. In a gastric cancer study, cholinergic signaling was found to facilitate neuron expansion and tumor development by virtue of upregulating NGF production and activating YAP (yes-associated protein 1) signaling, respectively [71]. Conversely, inhibition of parasympathetic input downregulated Wnt signaling and suppressed tumor stem cell expansion in a CHRM3-dependent fashion [72]. On the other hand, cholinergic input may also inhibit tumor progression. This was recently shown for pancreatic carcinoma in mice, in which subdiaphragmatic vagotomy or genetic knockout of CHRM1 accelerated oncogenesis, whereas cholinergic pathway activation following systemic administration of muscarinic agonist, bethanechol, suppressed tumor sternness [73]. These results underline the complexity of parasympathetic control of tumor progression and direct attention to CHRM3, which is involved in the prostate and gastric cancer models mentioned above. We found in ongoing, unpublished work that upregulation of the receptor-encoding gene, CHRM3, is associated with inferior survival in a large, publicly available database of patients with myeloma (Fig. 5). Similar associations were observed for three additional cholinergic receptor genes (CHRM2, CHRNA5, CHRNB4) but not for any of the βAR-encoding genes (results not shown). Intriguingly, a large body of epidemiologic evidence links occupational exposure to cholinergic compounds (pesticides) with increased incidence of MM [74,75]. This lends support to the possibility that parasympathetic signals are involved in myeloma development. GEMMs of human myeloma, including the one shown i: Fig. 4, may lend themselves to testing this hypothesis in a definitive manner, under genetically and environmentally controlled conditions not feasible for clinical trials.
Fig. 5.
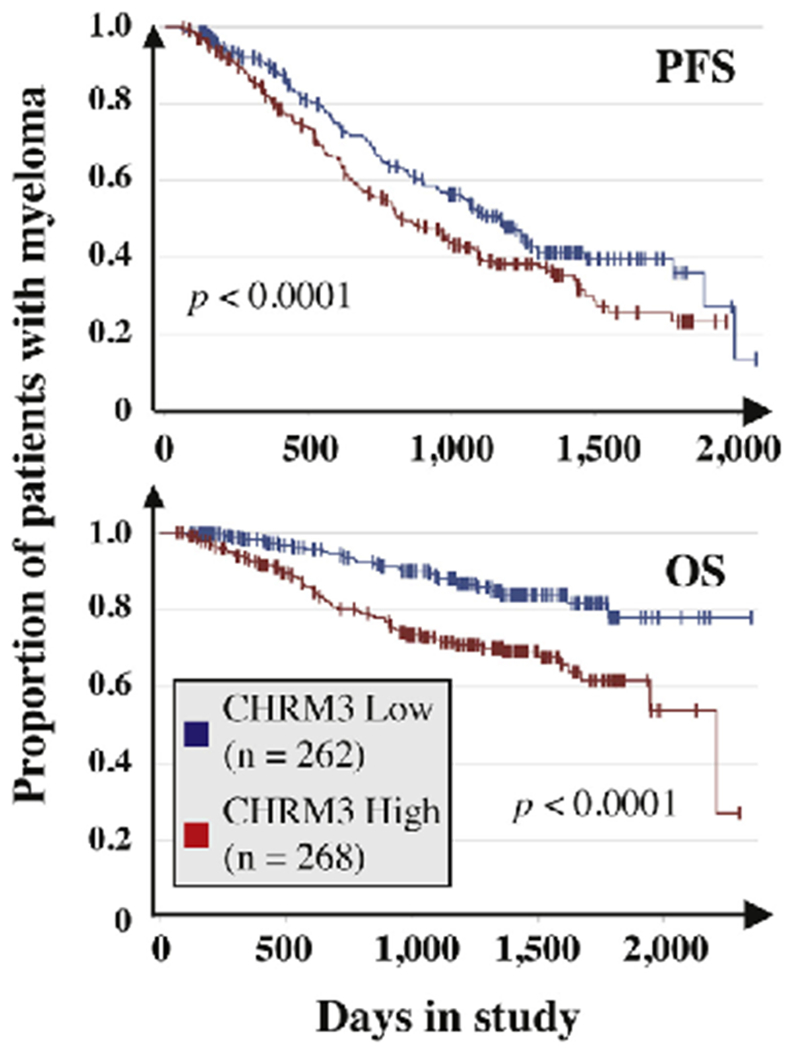
Elevated cholinergic receptor muscarinic 3 (CHRM3) mRNA level in myeloma cells predicts poor survival in the MMRF CoMMpass study. Kaplan-Meier curves of progression free survival (PFS) and overall survival (OS) are plotted. Censored patients are indicated by short vertical lines. Median gene expression was used as cutoff to allocate patients to the high expressor (red curve) or low expressor (blue curve) group. The number of patients in each group is shown. The results of log-rank analyses for differences in survival are also included. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
9. Summary and future directions
A growing body of evidence demonstrates the involvement of sympathetic (adrenergic) and parasympathetic (cholinergic) pathways of autonomic tissue control in the development and progression of solid tumors including breast, prostate, pancreatic, colon and stomach cancer [76]. In contrast, little is known about the significance of autonomic signaling in multiple myeloma (MM) and related plasma cell neoplasms. To remedy this shortcoming and enhance our understanding of ANS control of myeloma, a joint interdisciplinary collaborative research effort of expert oncologists and laboratory-based investigators from diverse fields of neuro- and cancer biology, genetics and immunology will be required. The clinical and epidemiologic results that are available at this juncture suggest that adrenergic input plays a role in the pathophysiology and outcome of myeloma. Chronic stress including anxiety and depression, going hand in hand with smoldering sympathetic (hyper)activation, have been repeatedly linked to elevated death rates in myeloma. In sync with that, a recent retrospective outcome analysis showed that anti-adrenergic β-blocker intake is associated with reduced risk of disease-specific mortality in patients with myeloma [5]. The beneficial effect of β- adrenergic receptor blockade is not unique to myeloma but has also been demonstrated in other cancers. For example, use of β1/β2 inhibitor, atenolol, for at least 3 years has been recently shown to lead to a significant reduction in odds of detecting clinically-significant intermediate and high-risk prostate cancer on initial prostate biopsy [77].
To develop a more complete understanding of the impact of the ANS on myeloma, it will be important in future studies to distinguish the direct crosstalk of autonomic nerves with tumor cells (using a synapse-like makeup?) from interactions with non-malignant bystander cells in the bone marrow microenvironment (BMM) such as immune, endothelial and bone cells. Indirect effects of ANS signaling may be highly relevant for MM, a prototype of a malignancy that exhibits exquisite dependence on a supportive tumor microenvironment (TME). Indirect ANS effects are important for normal plasma cell formation in mice, which requires B lymphocytes to express cholinergic nicotinic receptors that bind ACh supplied by NE-stimulated CD4+ T cells [78]. A challenging research task going forward concerns the possibility that ANS neurotransmitter (NE, ACh)-stimulated pathways regulate distinct stages of tumor development; e.g., the MGUS-to-MM transition early on as opposed to drug-resistant relapse at the terminal stage of disease progression. Another outstanding problem is to elucidate the role, if any, of parasympathetic signaling in myeloma. The somewhat surprising finding reported here that high message levels of cholinergic receptor genes in myeloma cells predict poor survival in the MMRF CoMMpass study suggests that cholinergic pathways promote myeloma progression. However, the reality that experimental data in support of this theory is lacking; the possibility that the elevation of receptor message is a simple compensatory change driven by some other autonomic dynamic (e.g., low cholinergic pathway activity due to low ACh ligand levels); and the preclinical result described in the previous section that cholinergic input inhibits pancreatic carcinoma in laboratory mice [73] all put us on guard to keep an open mind.
Practice points.
Chronic stress and depression, along with heightened sympathetic input, are prevalent in myeloma and associated with twofold risk elevation for all-cause mortality.
Myeloma patients may benefit from β-blockers, competitive small-compound inhibitors of β-adrenergic signaling.
Myeloma patients may benefit from stress relief, psychotherapy and interventions aimed at enhancing positive psychological resources.
Evidence that environmental and occupational exposure to cholinergic compounds promotes myelomagenesis suggests that conclusions that sympathetic activation and adrenergic signaling are the sole drivers of adverse ANS-dependent outcomes in myeloma are premature and oversimplified.
Enhanced understanding of the ANS-myeloma crosstalk is important because it may lead to the design and testing of new approaches to treat and prevent myeloma.
Cancer neuroscience, a burgeoning field that studies both the role of the nervous system in the natural history of cancer and the effect of cancer and cancer therapy on nervous system function [79], is relevant for myeloma.
Research agenda.
Assembling integrated bench-to-bedside-and-back research teams to assess the impact of autonomic tissue control on myeloma development, progression, and outcome.
Taking advantage of GEMMs of human myeloma to attack fundamental knowledge gaps on biological pathways and molecular mechanisms of ANS regulation of myeloma.
Determining mRNA and protein expression levels of adrenergic and cholinergic receptors in new and relapses myeloma and conducting biochemical studies on NE- and ACh-producing pathways in myeloma.
Translating insights into ANS control of normal HSC activity to new strategies for therapeutic targeting of multiple myeloma stem cells (MMSCs).
Assessing impact of peripheral neuropathy on myeloma bone disease (MBD).
Evaluating whether sensory nerves – which, just like their autonomic counterparts, belong to the peripheral nervous system (PNS) – act as more than conduits for MBD-related pain transmission in myeloma [80].
Designing clinical trials that target ANS-dependent pathways of myeloma treatment and progression to improve the outcome of myeloma.
Acknowledgments
Funding
This work was supported by the William G. Schuett, Jr., Multiple Myeloma Research Endowment (philanthropy, USA; awarded to SJ). Additional support was provided by K23 HL1414451 (National Heart, Lung, and Blood Institute, NIH, USA; awarded to AD) and R01 CA151354 (National Cancer Institute, NIH, USA; awarded to SJ).
Declaration of Competing Interest
AD received research funding from Takeda, TeneoBio, Sanofi and EDO Mundipharma and consulting fees from Pfizer, Akcea, Imbrium and Janssen. BD served on the advisory board of Takeda and Amgen and received honoraria from Celgene. SC served on the advisory board and received a honorarium from Takeda Pharmaceutical. HP received consulting fees and honoraria from BMS, Takeda, Amgen, Janssen, Karyopharm, Incyte, Sanofi and Abbvie. All other authors have no competing interests to declare.
Abbreviations:
- ACh
acetylcholine
- AChR
cholinergic receptor
- ANS
autonomic nervous system
- AR
adrenergic receptor
- BM
bone marrow
- BMM
bone marrow microenvironment
- ChAT
choline acetyltransferase
- CHRM
cholinergic muscarinic receptor
- HSC
hematopoietic stem cell
- HSPC
hematopoietic stem and progenitor cell
- MBD
myeloma bone disease
- MGUS
monoclonal gammopathy of undetermined significance
- MM
multiple myeloma
- NE
norepinephrine
- PSN
parasympathetic nervous system
- SNS
sympathetic nervous system
- TME
tumor microenvironment
References
- [1].Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009;113:5418–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Barlogie B, Mitchell A, van Rhee F, Epstein J, Morgan GJ, Crowley J. Curing myeloma at last: defining criteria and providing the evidence. Blood. 2014;124:3043–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ghobrial IM, Detappe A, Anderson KC, Steensma DP. The bone-marrow niche in MDS and MGUS: implications for AML and MM. Nat Rev Clin Oncol 2018;15:219–33. [DOI] [PubMed] [Google Scholar]
- [4].Mouhieddine TH, Weeks LD, Ghobrial IM. Monoclonal gammopathy of undetermined significance. Blood. 2019;133:2484–94. [DOI] [PubMed] [Google Scholar]
- [5].Hwa YL, Shi Q, Kumar SK, Lacy MQ, Gertz MA, Kapoor P, et al. Beta-blockers improve survival outcomes in patients with multiple myeloma: a retrospective evaluation. Am J Hematol 2017;92:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Knight JM, Kerswill SA, Hari P, Cole SW, Logan BR, D’Souza A, et al. Repurposing existing medications as cancer therapy: design and feasibility of a randomized pilot investigating propranolol administration in patients receiving hematopoietic cell transplantation. BMC Cancer 2018;18:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Knight JM, Rizzo JD, Hari P, Pasquini MC, Giles KE, D’Souza A, et al. Propranolol inhibits molecular risk markers in HCT recipients: a phase 2 randomized controlled biomarker trial. Blood Adv 2020;4:467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Al-Wadei HA, Al-Wadei MH, Schuller HM. Prevention of pancreatic cancer by the beta-blocker propranolol. Anticancer Drugs 2009;20:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chang P-Y, Huang W-Y, Lin C-L, Huang T-C, Wu Y-Y, Chen J-H, et al. Propranolol reduces cancer risk: a population-based cohort study. Medicine. 2015;94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol 2011;29:2635–44. [DOI] [PubMed] [Google Scholar]
- [11].Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med 2010;40:1797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Batty GD, Russ TC, Stamatakis E, Kivimaki M. Psychological distress in relation to site specific cancer mortality: pooling of unpublished data from 16 prospective cohort studies. BMJ. 2017;356:j108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mols F, Husson O, Roukema JA, van de Poll-Franse LV. Depressive symptoms are a risk factor for all-cause mortality: results from a prospective population-based study among 3,080 cancer survivors from the PROFILES registry. J Cancer Surviv 2013;7:484–92. [DOI] [PubMed] [Google Scholar]
- [14].Raphael D, Frey R, Gott M. Psychosocial distress in haematological cancer survivors: an integrative review. Eur J Cancer Care (Engl) 2017;26. [DOI] [PubMed] [Google Scholar]
- [15].Abuelgasim KA, Ahmed GY, Alqahtani JA, Alayed AM, Alaskar AS, Malik MA. Depression and anxiety in patients with hematological malignancies, prevalence, and associated factors. Saudi Med J 2016;37:877–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McFarland DC, Polizzi H, Mascarenhas J, Kremyanskaya M, Holland J, Hoffman R. Psychological symptoms among patients with BCR-ABL-negative myeloproliferative neoplasms. J Natl Compr Cane Netw 2016;14:1563–70. [DOI] [PubMed] [Google Scholar]
- [17].Wang ZY, Liu L, Shi M, Wang L. Exploring correlations between positive psychological resources and symptoms of psychological distress among hematological cancer patients: a cross-sectional study. Psychol Health Med 2016;21:571–82. [DOI] [PubMed] [Google Scholar]
- [18].Li Y, Yang Y, Zhang R, Yao K, Liu Z. The mediating role of mental adjustment in the relationship between perceived stress and depressive symptoms in hematological Cancer patients: a cross-sectional study. PLoS One 2015;10:e0142913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McFarland DC, Shen MJ, Polizzi H, Mascarenhas J, Kremyanskaya M, Holland J, et al. Preferences of patients with myeloproliferative neoplasms for accepting anxiety or depression treatment. Psychosomatics. 2017;58:56–63. [DOI] [PubMed] [Google Scholar]
- [20].Shreders AJ, Niazi SK, Hodge DO, Chimato NT, Kureti M, Kirla N, et al. Correlation of sociodemographic and clinical parameters with depression and distress in patients with hematologic malignancies. Ann Hematol 2018;97:519–28. [DOI] [PubMed] [Google Scholar]
- [21].Kozanoglu I, Yandim MK, Cincin ZB, Ozdogu H, Cakmakoglu B, Baran Y. New indication for therapeutic potential of an old well-known drug (propranolol) for multiple myeloma. J Cancer Res Clin Oncol 2013;139:327–35. [DOI] [PubMed] [Google Scholar]
- [22].Liu Y, Yu X, Zhuang J. Epinephrine stimulates cell proliferation and induces chemoresistance in myeloma cells through the beta-adrenoreceptor in vitro. Acta Haematol 2017;138:103–10. [DOI] [PubMed] [Google Scholar]
- [23].Yang EV, Donovan EL, Benson DM, Glaser R. VEGF is differentially regulated in multiple myeloma-derived cell lines by norepinephrine. Brain Behav Immun 2008;22:318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rickies RJ, Tam WF, Giordano TP 3rd, Pierce LT, Farwell M, McMillin DW, et al. Adenosine A2A and beta-2 adrenergic receptor agonists: novel selective and synergistic multiple myeloma targets discovered through systematic combination screening. Mol Cancer Ther 2012;11:1432–42. [DOI] [PubMed] [Google Scholar]
- [25].Xie B, Xu Z, Yang G, Chen G, Li B, Hu L, et al. Antitumor effect of dobutamine on multiple myeloma via mitogen-activated protein kinase pathway in vitro. Acta Biochim Biophys Sin (Shanghai) 2016;48:1135–7. [DOI] [PubMed] [Google Scholar]
- [26].Nakai A, Suzuki K. Adrenergic control of lymphocyte trafficking and adaptive immune responses. Neurochem Int 2019;130:104320. [DOI] [PubMed] [Google Scholar]
- [27].Jung WC, Levesque JP, Ruitenberg MJ. It takes nerve to fight back: the significance of neural innervation of the bone marrow and spleen for immune function. Semin Cell Dev Biol 2017;61:60–70. [DOI] [PubMed] [Google Scholar]
- [28].Kokolus KM, Zhang Y, Sivik JM, Schmeck C, Zhu J, Repasky EA, et al. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology. 2018;7:e1405205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res 2010;70:7042–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hou N, Zhang X, Zhao L, Zhao X, Li Z, Song T, et al. A novel chronic stress-induced shift in the Th1 to Th2 response promotes colon cancer growth. Biochem Biophys Res Commun 2013;439:471–6. [DOI] [PubMed] [Google Scholar]
- [31].Bucsek MJ, Qiao G, MacDonald CR, Giridharan T, Evans L, Niedzwecki B, et al. Beta-adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8(+) T cells and undermines checkpoint inhibitor therapy. Cancer Res 2017;77:5639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Elefteriou F Impact of the autonomic nervous system on the skeleton. Physiol Rev 2018;98:1083–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Toulis KA, Hemming K, Stergianos S, Nirantharakumar K, Bilezikian JP. beta-Adrenergic receptor antagonists and fracture risk: a meta-analysis of selectivity, gender, and site-specific effects. Osteoporos Int 2014;25:121–9. [DOI] [PubMed] [Google Scholar]
- [34].Olechnowicz SW, Edwards CM. Contributions of the host microenvironment to cancer-induced bone disease. Cancer Res 2014;74:1625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Delforge M, Blade J, Dimopoulos MA, Facon T, Kropff M, Ludwig H, et al. Treatment-related peripheral neuropathy in multiple myeloma: the challenge continues. Lancet Oncol 2010;11:1086–95. [DOI] [PubMed] [Google Scholar]
- [36].Azoulay D, Lavie D, Horowitz N, Suriu C, Gatt ME, Akria L, et al. Bortezomib-induced peripheral neuropathy is related to altered levels of brain-derived neurotrophic factor in the peripheral blood of patients with multiple myeloma. Br J Haematol 2014;164:454–6. [DOI] [PubMed] [Google Scholar]
- [37].Lucas D, Scheiermann C, Chow A, Kunisaki Y, Bruns I, Barrick C, et al. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic re-generation. Nat Med 2013;19:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ai LS, Sun CY, Wang YD, Zhang L, Chu ZB, Qin Y, et al. Gene silencing of the BDNF/TrkB axis in multiple myeloma blocks bone destruction and tumor burden in vitro and in vivo. Int J Cancer 2013;133:1074–84. [DOI] [PubMed] [Google Scholar]
- [39].Azoulay D, Giryes S, Nasser R, Sharon R, Horowitz NA. Prediction of chemotherapy-induced peripheral neuropathy in patients with lymphoma and myeloma: the roles of brain-derived neurotropic factor protein levels and a gene polymorphism. J Clin Neurol 2019;15:511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang H, Zhang H, Srinivasan V, Tao J, Sim W, Lin X, et al. Targeting bortezomib to bone increases its bone anabolic activity and reduces systemic adverse effects in mice. J Bone Miner Res 2020;35:343–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol 2019;20:303–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Johnsen HE, Bogsted M, Schmitz A, Bodker JS, El-Galaly TC, Johansen P, et al. The myeloma stem cell concept, revisited: from phenomenology to operational terms. Haematologica. 2016;101:1451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wei Q, Frenette PS. Niches for hematopoietic stem cells and their progeny. Immunity. 2018;48:632–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gao X, Xu C, Asada N, Frenette PS. The hematopoietic stem cell niche: from embryo to adult. Development. 2018;145:dev139691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chartier SR, Mitchell SAT, Majuta LA, Mantyh PW. The changing sensory and sympathetic innervation of the young, adult and aging mouse femur. Neuroscience 2018;387:178–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Maryanovich M, Zahalka AH, Pierce H, Pinho S, Nakahara F, Asada N, et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat Med 2018;24:782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cui B, Luo Y, Tian P, Peng F, Lu J, Yang Y, et al. Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J Clin Invest 2019;129:1030–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Decker AM, Jung Y, Cackowski FC, Yumoto K, Wang J, Taichman RS. Sympathetic signaling reactivates quiescent disseminated prostate cancer cells in the bone marrow. Mol Cancer Res 2017;15:1644–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhang B, Ma S, Rachmin I, He M, Baral P, Choi S, et al. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature. 2020;577:676–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–21. [DOI] [PubMed] [Google Scholar]
- [51].Mendez-Ferrer S, Battista M, Frenette PS. Cooperation of beta(2)- and beta(3)-adrenergic receptors in hematopoietic progenitor cell mobilization. Ann N Y Acad Sci 2010;1192:139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ghobrial IM, Liu CJ, Zavidij O, Azab AK, Baz R, Laubach JP, et al. Phase I/II trial of the CXCR4 inhibitor plerixafor in combination with bortezomib as a chemosensitization strategy in relapsed/refractory multiple myeloma. Am J Hematol 2019;94:1244–53. [DOI] [PubMed] [Google Scholar]
- [53].Ghobrial IM, Liu CJ, Redd RA, Perez RP, Baz R, Zavidij O, et al. A phase Ib/II trial of the first-in-class anti-CXCR4 antibody ulocuplumab in combination with lenalidomide or bortezomib plus dexamethasone in relapsed multiple myeloma. Clin Cancer Res 2020;26:344–53. [DOI] [PubMed] [Google Scholar]
- [54].Lucas D, Bruns I, Battista M, Mendez-Ferrer S, Magnon C, Kunisaki Y, et al. Norepinephrine reuptake inhibition promotes mobilization in mice: potential impact to rescue low stem cell yields. Blood. 2012;119:3962–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shastri A, Budhathoki A, Barta SK, Komblum N, Derman O, Battini R, et al. Stimulation of adrenergic activity by desipramine enhances hematopoietic stem and progenitor cell mobilization along with G-CSF in multiple myeloma: a pilot study. Am J Hematol 2017;92:1047–51. [DOI] [PubMed] [Google Scholar]
- [56].Jobling P, Pundavela J, Oliveira SM, Roselli S, Walker MM, Hondermarck H. Nerve–cancer cell cross-talk: a novel promoter of tumor progression. Cancer Res 2015;75:1777–81. [DOI] [PubMed] [Google Scholar]
- [57].Faulkner S, Jobling P, March B, Jiang CC, Hondermarck H. Tumor neurobiology and the war of nerves in cancer. Cancer Discov 2019;9:702–10. [DOI] [PubMed] [Google Scholar]
- [58].Lamkin DM, Sloan EK, Patel AJ, Chiang BS, Pimentel MA, Ma JC, et al. Chronic stress enhances progression of acute lymphoblastic leukemia via beta-adrenergic signaling. Brain Behav Immun 2012;26:635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Harms P, Paczulla AM, Medinger M, Konantz M, Lengerke C. Stress and catecholamines modulate the bone marrow microenvironment to promote tumorigenesis. Cell Stress 2019;3:221–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 2006;12:939–44. [DOI] [PubMed] [Google Scholar]
- [61].Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. [DOI] [PubMed] [Google Scholar]
- [62].Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res 2012;18:1201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Braadland PR, Ramberg HA, Grytli HH, Taskén KA. β-adrenergic receptor signaling in prostate cancer. Front Oncol 2015;4:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Huan HB, Wen XD, Chen XJ, Wu L, Wu LL, Zhang L, et al. Sympathetic nervous system promotes hepatocarcinogenesis by modulating inflammation through activation of alphal-adrenergic receptors of Kupffer cells. Brain Behav Immun 2017;59:118–34. [DOI] [PubMed] [Google Scholar]
- [65].Zahalka AH, Amal-Estape A, Maryanovich M, Nakahara F, Cruz CD, Finley LWS, et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science. 2017;358:321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Renz BW, Takahashi R, Tanaka T, Macchini M, Hayakawa Y, Dantes Z, et al. beta2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell 2018;33:75–90. [ e7]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Madeo M, Colbert PL, Vermeer DW, Lucido CT, Cain JT, Vichaya EG, et al. Cancer exosomes induce tumor innervation. Nat Commun 2018;9:4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Arranz L, Sanchez-Aguilera A, Martin-Perez D, Isem J, Langa X, Tzankov A, et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512:78–81. [DOI] [PubMed] [Google Scholar]
- [69].Hanoun M, Zhang D, Mizoguchi T, Pinho S, Pierce H, Kunisaki Y, et al. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell 2014;15:365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wang N, Yao M, Xu J, Quan Y, Zhang K, Yang R, et al. Autocrine activation of CHRM3 promotes prostate cancer growth and castration resistance via CaM/CaMKK-mediated phosphorylation of Akt. Clin Cancer Res 2015;21:4676–85. [DOI] [PubMed] [Google Scholar]
- [71].Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell 2017;31:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med 2014;6:250ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Renz BW, Tanaka T, Sunagawa M, Takahashi R, Jiang Z, Macchini M, et al. Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer sternness. Cancer Discov 2018;8:1458–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Tual S, Busson A, Boulanger M, Renier M, Piel C, Pouchieu C, et al. Occupational exposure to pesticides and multiple myeloma in the AGRICAN cohort. Cancer Causes Control 2019;30:1243–50. [DOI] [PubMed] [Google Scholar]
- [75].Shearer JJ, Beane Freeman LE, Liu D, Andreotti G, Hamilton J, Happel J, et al. Longitudinal investigation of haematological alterations among permethrin-ex-posed pesticide applicators in the biomarkers of exposure and effect in agriculture study. Occup Environ Med 2019;76:467–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zahalka AH, Frenette PS. Nerves in cancer. Nat Rev Cancer 2020;20:143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zahalka AH, Fram E, Lin W, Mohn L, Frenette PS, Agalliu I, et al. Use of beta-blocker types and risk of incident prostate cancer in a multiethnic population. Urol Oncol 2020. 10.1016/j.urolonc.2020.03.024. In press. [DOI] [PubMed] [Google Scholar]
- [78].Zhang X, Lei B, Yuan Y, Zhang L, Hu L, Jin S, et al. Brain control of humoral immune responses amenable to behavioural modulation. Nature. 2020;581:204–8. [DOI] [PubMed] [Google Scholar]
- [79].Monje M, Borniger JC, D’Silva NJ, Deneen B, Dirks PB, Fattahi F, et al. Roadmap for the emerging field of cancer neuroscience. Cell. 2020;181:219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Saloman JL, Albers KM, Rhim AD, Davis BM. Can stopping nerves, stop cancer? Trends Neurosci 2016;39:880–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bjurholm A, Kreicbergs A, Terenius L, Goldstein M, Schultzberg M. Neuropeptide Y-, tyrosine hydroxylase-and vasoactive intestinal polypeptide-immunoreactive nerves in bone and surrounding tissues. J Auton Nerv Syst 1988;25:119–25. [DOI] [PubMed] [Google Scholar]
- [82].Tabarowski Z, Gibson-Berry K, Felten SY. Noradrenergic and peptidergic innervation of the mouse femur bone marrow. Acta Histochem 1996;98:453–7. [DOI] [PubMed] [Google Scholar]
- [83].Artico M, Bosco S, Cavallotti C, Agostinelli E, Giuliani-Piccari G, Sciorio S, et al. Noradrenergic and cholinergic innervation of the bone marrow. Int J Mol Med 2002;10:77–80. [PubMed] [Google Scholar]
- [84].Bajayo A, Bar A, Denes A, Bachar M, Kram V, Attar-Namdar M, et al. Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual. Proc Natl Acad Sci 2012;109:15455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer 2015;15:563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lu D, Sinnott JA, Valdimarsdottir U, Fang F, Gerke T, Tyekucheva S, et al. Stress-related signaling pathways in lethal and nonlethal prostate cancer. Clin Cancer Res 2016;22:765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Cole SW. New challenges in psycho-oncology: neural regulation of the cancer genome. Psychooncology. 2018;27:2305–9. [DOI] [PubMed] [Google Scholar]
- [88].Cole SW. The conserved transcriptional response to adversity. Curr Opin Behav Sci 2019;28:31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Chen H, Liu D, Guo L, Cheng X, Guo N, Shi M. Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating beta-adrenergic signaling. J Pathol 2018;244:49–60. [DOI] [PubMed] [Google Scholar]
- [90].Saul AN, Oberyszyn TM, Daugherty C, Kusewitt D, Jones S, Jewell S, et al. Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst 2005;97:1760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hassan S, Karpova Y, Baiz D, Yancey D, Pullikuth A, Flores A, et al. Behavioral stress accelerates prostate cancer development in mice. J Clin Invest 2013;123:874–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Feng Z, Liu L, Zhang C, Zheng T, Wang J, Lin M, et al. Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc Natl Acad Sci USA 2012;109:7013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Cao L, Liu X, Lin EJ, Wang C, Choi EY, Riban V, et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010;142:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hanoun M, Maryanovich M, Amal-Estapé A, Frenette PS. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron. 2015;86:360–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Corr A, Smith J, Baldock P. Neuronal control of bone remodeling. Toxicol Pathol 2017;45:894–903. [DOI] [PubMed] [Google Scholar]
- [96].Togari A, Arai M, Kondo A. The role of the sympathetic nervous system in controlling bone metabolism. Expert Opin Ther Targets 2005;9:931–40. [DOI] [PubMed] [Google Scholar]
- [97].Dimitri P, Rosen C. The central nervous system and bone metabolism: an evolving story. Calcif Tissue Int 2017;100:476–85. [DOI] [PubMed] [Google Scholar]
- [98].Tompkins VS, Rosean TR, Holman CJ, DeHoedt C, Olivier AK, Duncan KM, et al. Adoptive B-cell transfer mouse model of human myeloma. Leukemia. 2016;30:962–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


