Fig. 4.
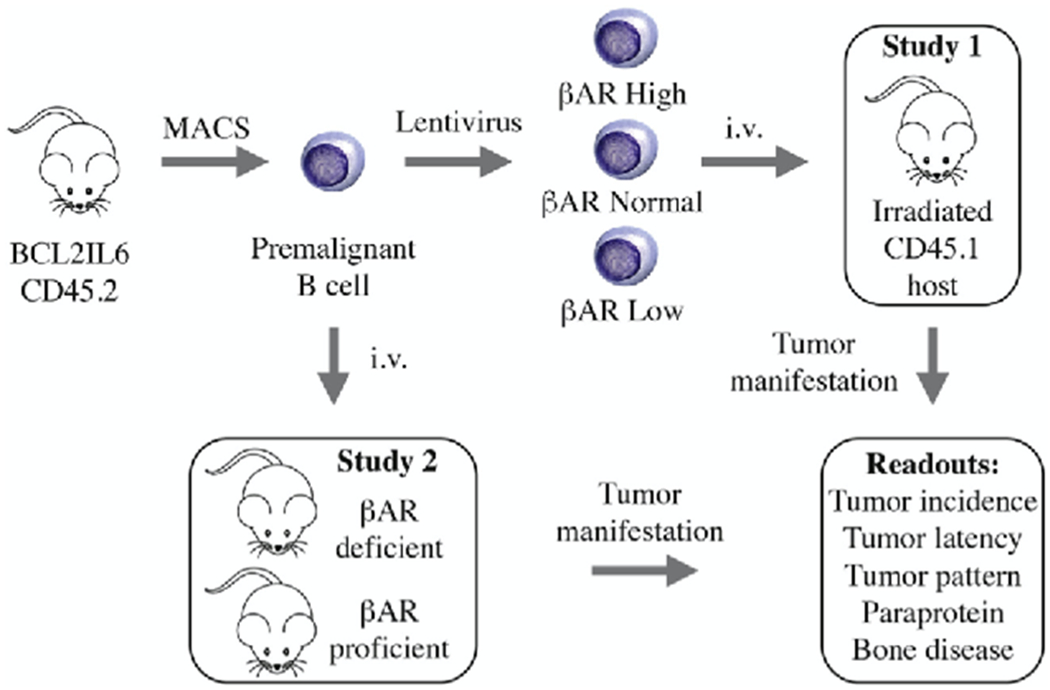
Experimental strategy to evaluate the role of adrenergic signaling in plasma cell tumor (PCT) development in mice. Adoptive B-cell transfer provides a flexible platform for assessing role and significance of adrenergic input in the natural history of myeloma-like PCTs. Effects on tumor precursors (Study 1) can be distinguished from those on the TME (Study 2), as recently shown in a study on the impact of IL-6 (interleukin 6) on PCT development [98]. Briefly, donor B cells are isolated from double-transgenic BCL2IL6 mice on the genetic background of BALB/c, using MACS® magnetic bead columns (cartoon to the upper left). Next, B cells are genetically modified in short-term cell culture using lentiviral gene transduction, which results in enforced expression of a βAR gene of interest (βAR High) or RNAi-mediated downregulation of βAR expression (βAR Low). Transfection with non-coding “empty” virus that leaves endogenous βAR expression unchanged is used as control (βAR Normal). Adoptive transfer of transfected B cells to sub-lethally irradiated hosts congenic for CD45.1 generates 3 cohorts of mice distinguished only by the level of βAR message in tumor precursors (upper right). Tumor incidence, latency and pattern will serve as endpoints of the study. Specific antibodies for the two CD45 allotypes involved provide a convenient tool for monitoring engraftment and neoplastic expansion of donor cells in host tissues, using flow cytometry and immunohistochemistry as measurement tools. Transfer tumor precursors to host mice that are either deficient or proficient in βAR signaling (Study 2) may show whether βAR signaling in the TME is critical for PCT development.
