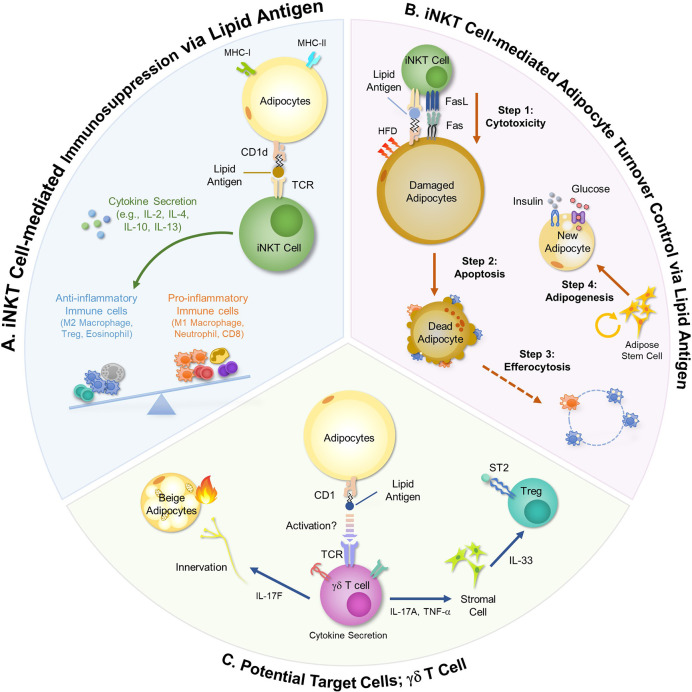
Figure 1.
Immunomodulatory Roles of Adipocytes using Lipid Antigens. Adipocytes modulate activities of adipose immune cells via lipid antigen presentation. iNKT cells and γδ T cells are activated by lipid antigens and involve in the regulation of adipose tissue immunity and adipocyte functions. (A) In obesity, adipose iNKT cells activated by adipocyte-derived lipid antigens secret large amounts of anti-inflammatory cytokines such as IL-2, IL-4, IL-10, and IL-13. These cytokines stimulate Treg cells and polarize monocytes into anti-inflammatory M2 macrophages, thereby ameliorating pro-inflammatory responses in obese adipose tissue. (B) Adipose iNKT cells mediate hypertrophic and pro-inflammatory adipocyte death in obesity. Long-term HFD (over 8 weeks) upregulates CD95L (FasL) and CD95 (Fas) in adipose iNKT cells and damaged adipocytes, respectively. Interaction between CD95L and CD95 selectively stimulates damaged adipocyte death. After macrophage-mediated efferocytosis, adipose stem cells proliferate and de novo adipogenesis is promoted, leading to the generation of insulin-sensitive new adipocytes. (C) Given that γδ T cells recognize CD1-loaded lipid antigens, it has been suggested that adipocytes might regulate γδ T cell activity. γδ T cells secrete several cytokines such as IL-17 and TNF-α, controlling beige adipocyte formation and innervation. In addition, γδ T cells activate stromal cells to secrete IL-33, resulting in Treg cell recruitment.