Figure 2.
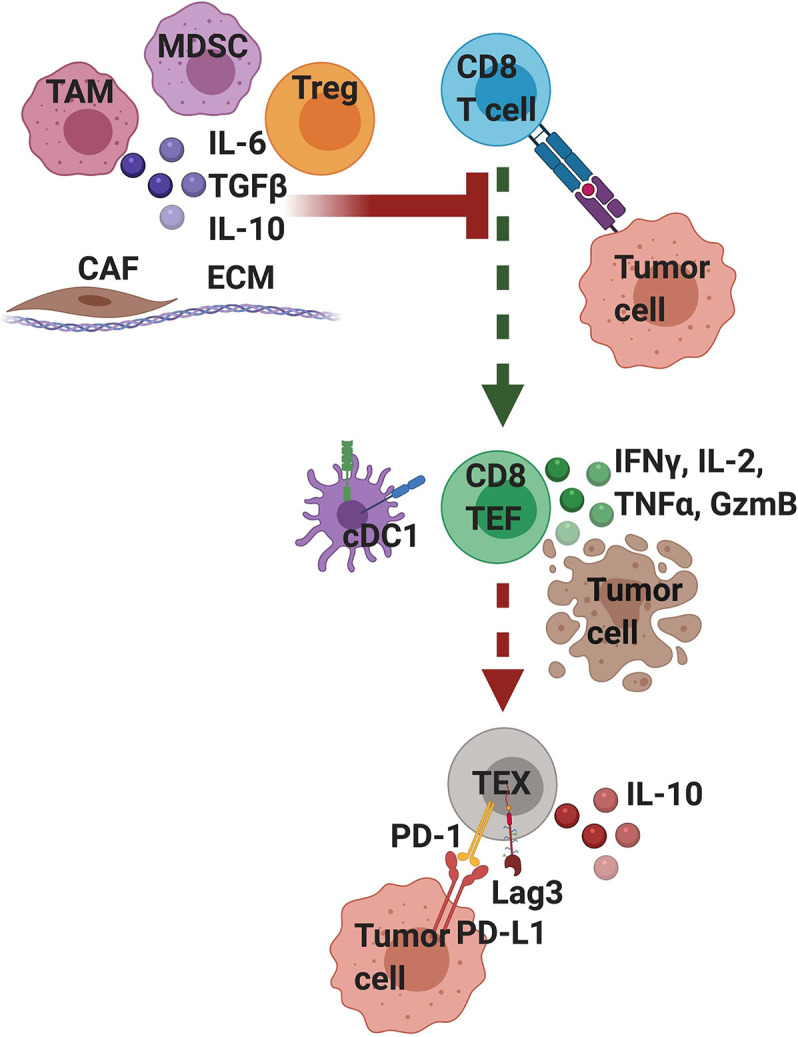
T cell intrinsic and extrinsic factors influence T cell differentiation and functionality in pancreatic cancer. Tumor-infiltrating T cells that strongly recognize tumor antigen mediate transient anti-tumor activity but if the tumor is not cleared, differentiate into exhausted (TEX) T cells, which is driven by persistent T cell receptor (TCR) signaling. Exhausted T cells are often characterized by their expression of PD-1 and Lag3 and are hypofunctional. Tumor cells and other stromal cells can express ligands for these inhibitory receptors, such as PD-L1, a ligand for PD- 1. Signaling through these inhibitory receptors interferes with T cell function and differentiation state. Moreover, exhausted T cells may participate in their own suppression by producing IL-10. A variety of extrinsic cells and factors enriched in the suppressive tumor microenvironment can interfere with TCR signaling and activation. These include tumor associated macrophages (TAM), myeloid-derived suppressor cells (MDSC), regulatory T cells (Tregs), and cancer associated fibroblasts (CAF) all embedded within a dense extracellular matrix (ECM). Immunosuppressive cells secrete cytokines including IL-10 and TGFβ that can suppress T cell activation and TCR signaling. Mechanistically, extrinsic suppression and TCR-driven exhaustion are distinct processes that converge to suppress tumor immunity. We posit that targeting immunosuppression may lead to only transient anti-tumor immune responses because if the tumor is not cleared; T cells will become exhausted. Thus, PDA likely requires combination immunotherapies that target both T cell extrinsic immunosuppression and T cell intrinsic exhaustion.
