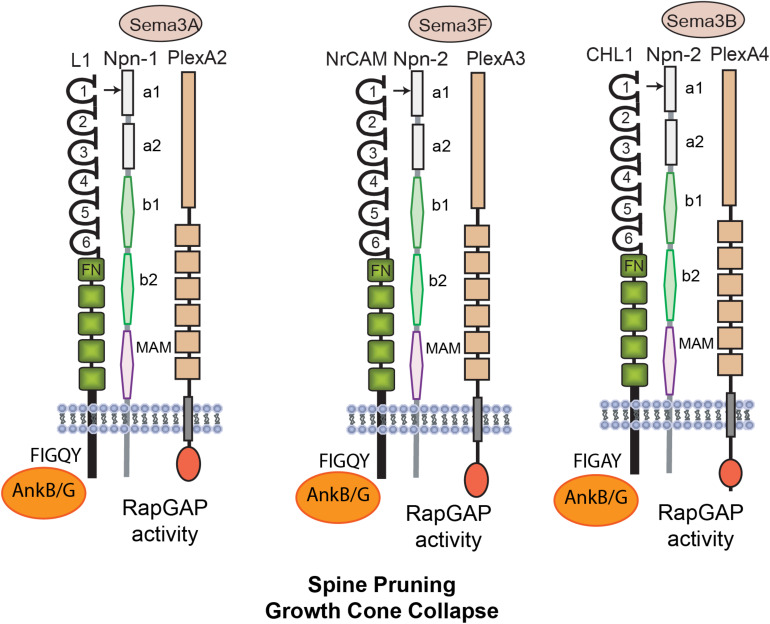
FIGURE 1.
Mechanisms of L1 Family Mediated Spine Pruning. L1 is a transmembrane glycoprotein with 6 Ig and 5 FNIII domains and a short cytoplasmic tail. Homophilic binding in cis and trans is mediated principally by the Ig2 domain, which is present within a folded horseshoe conformation of Ig1-4. The L1 Ig1 domain binds heterophilically to the Sema3A co-receptor Neuropilin-1 (Npn1). Npn1/2 consist of 2 CUB domains (a1, a2), 2 coagulation factor V/VII domains (b1, b2) and a meprin-A5-mu domain (MAM). Other L1-CAMs Close Homolog of L1 (CHL1) and NrCAM have similar structures and carry out related functions. The NrCAM Ig1 domain constitutively binds Npn2 at its a1 domain and mediates responses to Sema3F in complex with PlexA3. Activation of the intrinsic Rap-GAP activity of PlexAs downregulates Rap1-GTPase. CHL1 binds Npn2 and mediates similar responses to Sema3B in complex with PlexA4. All L1-CAMs reversibly bind AnkyrinB/G (AnkB/G), a spectrin-actin adaptor protein, at a conserved motif FIGQ/AY in the cytoplasmic domain.