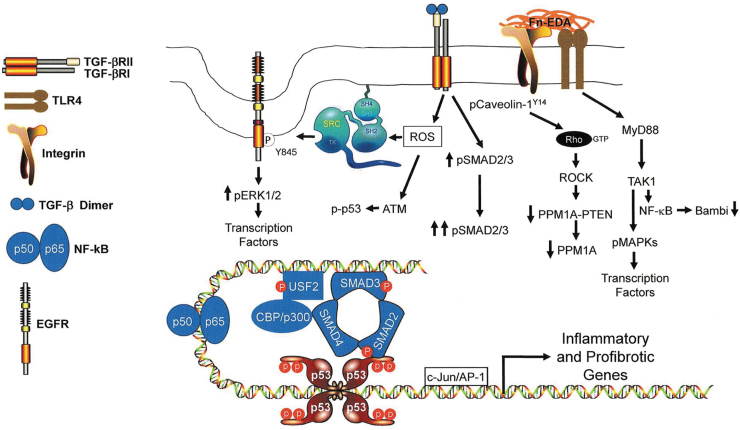
Figure 5.
Model illustrating TLR4-dependent signaling events that impact the expression of inflammatory and profibrotic genes. EDA stimulation of TLR4 signaling, perhaps in cooperation with the α4β1 integrin, engages the MyD88 adaptor protein pathway mobilizing TGF-β-activated kinase 1 (TAK1), also known as MAP3K7. TAK1, in turn, activates NF-κB resulting in the NF-κB-mediated downregulation of Bambi,156,157 enhancing thereby TGF-β signaling while increasing NF-κB(p50/p65)-dependent transcription of inflammatory genes. Bambi suppression, particularly in the context of increased TGF-β synthesis and/or release enhances TGF-βR-dependent SMAD2/3 phosphorylation while increasing the cellular levels of ROS. ROS signaling stimulates ATM-induced p53 phosphorylation, src kinase transactivation of the EGFR at Y845 and src-dependent phosphorylation of caveolin-1 at the Y14 site.142 EGFR-activated ERK1/2 and TAK1-stimulated MAPKs target transcription factors (e.g., NF-κB, USF, AP-1) and chromatin remodeling proteins (e.g., CB/p300) that cooperate with SMADs and p53 to influence expression of a genomic proinflammatory/profibrotic program. TGF-β1 also activates the RhoA/ROCK pathway, likely by promoting src kinase-induced caveolin-1 Y14 phosphorylation and Rho-GTP loading142 that downregulates both PTEN and PPM1A levels contributing to the persistence of SMAD2/3 phosphorylation and transcription of profibrotic genes. ROS, reactive oxygen species.