Fig. 2.
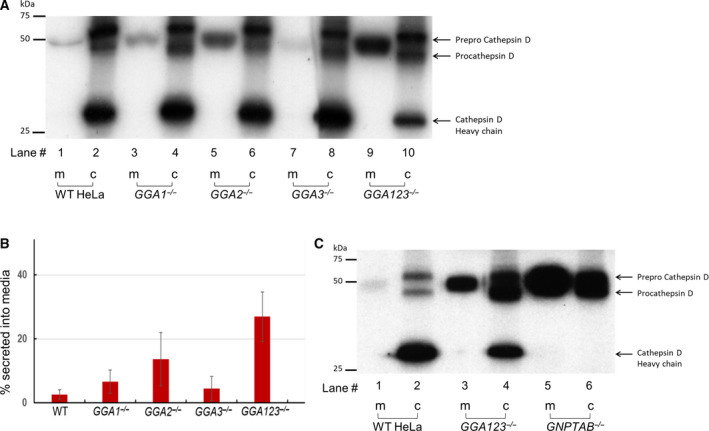
Sorting of cathepsin D in the absence of GGA proteins. (A) Parental WT, GGA1−/−, GGA2−/−, GGA3−/−, and GGA123−/− HeLa cell lines were metabolically labeled with [35S]methionine/cysteine and processed as described under ‘Materials and methods’. Secreted and intracellular cathepsin D molecules were immunoprecipitated with a polyclonal anti‐cathepsin D antibody, resolved by 10% SDS/PAGE and visualized by autoradiography of the dried gel. A representative autoradiograph is shown. (B) The percentage of cathepsin D in the media (m) was calculated as the ratio of radioactivity in the secreted form divided by the sum of the processed (c) and secreted forms (m). The data shown are the mean ± SD for four independent experiments. (C) Parental WT, GGA123−/−, and GNPTAB−/− HeLa cell lines were processed for cathepsin D sorting as described in (A). A representative autoradiograph is shown, and data presented are the mean of two independent experiments.
