Abstract
Metals and alloys, including stainless steel, titanium and its alloys, cobalt alloys, and other metals and alloys have been widely used clinically as implant materials, but implant-related infection or inflammation is still one of the main causes of implantation failure. The bacterial infection or inflammation that seriously threatens human health has already become a worldwide complaint. Antibacterial metals and alloys recently have attracted wide attention for their long-term stable antibacterial ability, good mechanical properties and good biocompatibility in vitro and in vivo. In this review, common antibacterial alloying elements, antibacterial standards and testing methods were introduced. Recent developments in the design and manufacturing of antibacterial metal alloys containing various antibacterial agents were described in detail, including antibacterial stainless steel, antibacterial titanium alloy, antibacterial zinc and alloy, antibacterial magnesium and alloy, antibacterial cobalt alloy, and other antibacterial metals and alloys. Researches on the antibacterial properties, mechanical properties, corrosion resistance and biocompatibility of antibacterial metals and alloys have been summarized in detail for the first time. It is hoped that this review could help researchers understand the development of antibacterial alloys in a timely manner, thereby could promote the development of antibacterial metal alloys and the clinical application.
Keywords: Antibacterial metals and alloys, Antibacterial alloying elements, Antibacterial stainless steel, Antibacterial titanium alloy, Antibacterial magnesium alloy
Graphical abstract
Highlights
-
•
This paper focuses the recent development of several antibacterial metals and alloys as biomedical materials.
-
•
The possible antibacterial mechanisms of antibacterial metals and alloys are summarized in this paper.
-
•
This review discusses the feasibility of antibacterial metals and alloys as biomedical implants in the future.
1. Introduction
Metals and alloys, including stainless steel, titanium and its alloys and cobalt alloys, have been widely used clinically due to their high strength, good wear resistance, good corrosion resistance, high fatigue properties and good biocompatibility, such as dental implants, hip and knee replacements, bone plates and screws. It was reported that up to 2.5 million dollars medical devices were made of metals and alloys.
The medical devices greatly improve the life quality of patients, but the device related infection or inflammation is still a main complaint despite strict antiseptic operating procedures, including systemic prophylaxis. It was reported that surgical site infection represents the most common nosocomial infection, accounting for 15% of all nosocomial infections [1]. Oral permucosal implants pose a very high risk of infection, as they breach the epithelial barrier and are thereby permanently exposed to the oral microflora. It was reported that 90% of all implants showed signs of inflammation and 50% of all implants showed signs of irreversible tissue destruction [2]. Nevertheless, the ten-year survival rates of endosseus implants were reported to be around 90–96% [2]. For dental implants, the most frequent cause of failure of dental implants is peri-implant inflammatory diseases such as peri-implantitis, which normally causes a circumferential bone loss and in turn may compromise the longevity of an implant. An analysis based on 11 studies showed a mean prevalence of peri-implantitis of 22% and a positive relationship between insertion time and prevalence [3] in which 5–11% of dental implants failed and must be removed [4]. Peri-implantitis affected about 10% of implants and 20% of patients during a 5–10 year observation period after implant placement [5]. For orthopedic replacement, with sterile operation and perioperative antibiotic defenses, the risk of infection after internal fixation ranged between 0.4% and 16.1% depending on the extent of fracture [6,7]. To be more precise, the infection rates of periprosthetic joint infections were 0.5%–2% [8], 2%–9% [9], and 0.3%–1.7% [10] after total joint replacement of the knee, hip and ankle, respectively.
The bacterial infection or inflammation that seriously threatens human health has already become a worldwide complaining issue [11]. So research on antibacterial technologies [12], including mechanical clearance, surface modification [13] and antibacterial materials is urgently necessary. Taking dental implants for example, the biofilm has to be removed mechanically, which should not change the micromorphology of the implant surface. Mechanical in vitro and in vivo treatments with an air powder abrasion device or with a titanium brush left around 10% bacterial residues on rough sand blasted titanium surfaces [14,15]. Surface modification can effectively prohibit the biofilm formation on the implant materials, such as developing a surface which causes cell damage to the adhered bacteria or anti-adhesive properties that inhibits bacterial adhesion in the first place. Various approaches have been used to convert the surfaces of biomedical devices into antimicrobial surfaces and lately reviews have been published on these approaches [4].
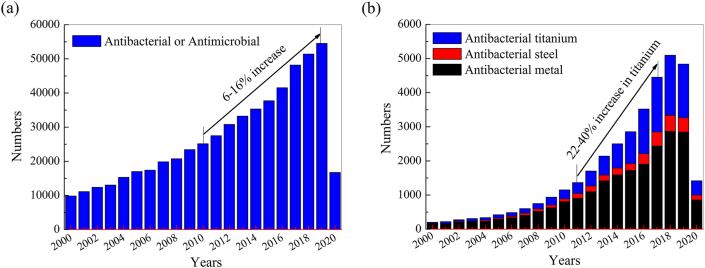
Antibacterial metals and alloys are a kind of metallic materials, which exhibits strong ability to inhibit the adhesion, the growth and the proliferation of a bacterium by element alloying and proper metal forming and heat treatment. Recently, Cu- and Ag-containing antibacterial metal alloys have been reported to exhibit good antibacterial ability against lots of bacteria, such as antibacterial stainless steel [16], antibacterial titanium [17,18], antibacterial magnesium and alloys [19] and antibacterial cobalt alloy [20,21]. The alloying elements mainly are Ag and Cu elements, which have been reported to have broad spectrum antibacterial ability. Nowadays, antibacterial metal alloys have attracted much attention worldwide and lots of new progresses have been achieved, which will promote the clinical application of antibacterial metal alloys. Fig. 1 shows the publication numbers on antibacterial or antimicrobial research searched by Web of Science. There are about 56,000 publications on this area in the past two decades with a 6–16% increase in the past decade. But research on “Antibacterial Metal” shows a much higher increase, especially on “Antibacterial Titanium”, which even increased by as much as 40% in 2016.
Fig. 1.
Publications on antibacterial research searched by Web of Science. a) key words: antibacterial or antimicrobial in topic, b) keywords: antibacterial metal, antibacterial steel and antibacterial titanium in topic. (Updated at 2020-05-21).
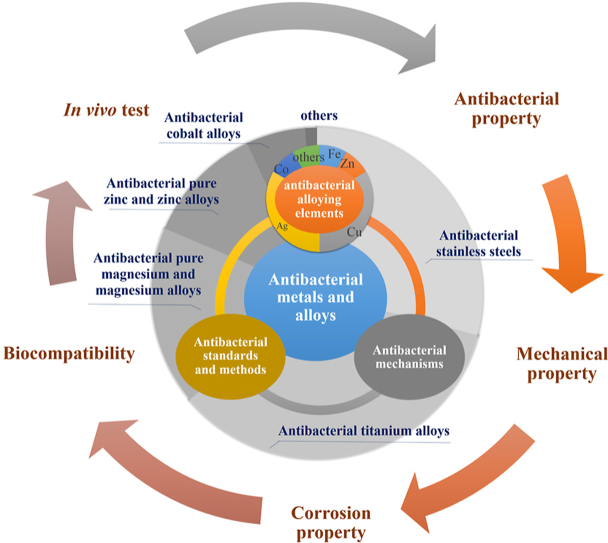
This paper summarizes the recent research results on antibacterial metals and alloys for the first time and Fig. 2 overviews the main framework of this review. It is hoped this review could benefit the flourishing development of antibacterial metals and metal alloys.
Fig. 2.
The main framework of this review.
2. Interaction between bacterium and metal alloy
In order to develop antibacterial metals and alloys, it is very necessary to understand the interaction between a bacterium and materials. The interaction between a bacterium and materials, such as metallic materials can be roughly divided into four steps:
-
1)
The primary step is the bacterial adhesion to a material surface. As pointed out by Campoccia [12], bacterial adhesion on materials surfaces is critically influenced by numerous variables, including the type of pathogen, the nature of physiological fluids, the surface morphometry and the surface physico-chemical properties of materials. The bacterial adhesion process is reversible.
-
2)
The secondary step is the colonization of the bacterium on the material surface. During this process, bacteria accumulate on the surface and colonize. The colonization is influenced by specific molecular and cellular interaction (adhesion proteins, proteinaceous appendages and extracellular polymeric substance production) [22]. On the other hand, bacterial colonization also changes the surface chemistry of the substrate by virtue of its metabolic byproducts. This process is irreversible.
-
3)
The third step is the biofilm formation and maturation. When bacteria colonize on the surface, bacterial microcolonies will form and bacteria will produce exopolymeric substances (mainly polysaccharides and other macromolecules), which contribute to biofilm formation. Once a biofilm is formed on the surface, it will protect bacteria in self-produced polysaccharidic matrix from both fluid shear stress and the action of systemic pharmacological therapies [22].
-
4)
The final step is the proliferation of bacteria. With the protection of the biofilm, bacteria begin to proliferate on the surface. As a result, the whole surface will be covered by bacteria.
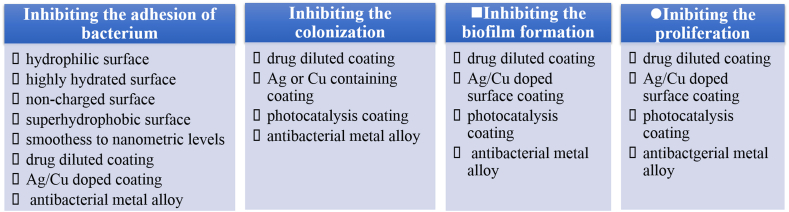
From the interaction between a bacterium and materials, it can be easily deduced that the strategies to reduce bacteria can be also roughly divided into four steps corresponding to the above four-step interaction, as shown in Fig. 3:
-
1)
Strategies to inhibit the adhesion of a bacterium on a metal surface, or anti-adhesion.
-
2)
Strategies to inhibit the colonization of a bacterium on a metal surface, or anti-colonization.
-
3)
Strategies to inhibit the biofilm formation on a metal surface, or anti-biofilm.
-
4)
Strategies to inhibit the proliferation of a bacterium on a metal surface, or anti-proliferation.
Fig. 3.
Antibacterial Strategies for metal alloys.
As stated above, the biofilm will protect the bacteria from external adversity. Therefore, the strategies mainly focus on the inhibition of the bacterium adhesion, the colonization and the biofilm formation. Lots of factors affect the bacteria adhesion, including the surface morphometry and physical-chemical properties of materials, environmental conditions as well as pathogen. Many surface technologies have been developed to change the surface properties, such as polymer coating, drug-eluting coating and charged surface [12]. Low adhesiveness of antifouling surfaces could possibly hinder tissue adhesion and integration of the implant. Nanostructure surface topography can determine a different bacterial behavior not just in terms of adhesion, but also in terms of cell metabolism, finally resulting bioactive. Smoothness down to nanometric levels was found to be associated in vitro to the lowest adhesion of both Gram-positive and Gram-negative bacteria [23].
It has been demonstrated that Ag- or Cu-containing surface coatings could resist the cell adhesion, the colonization as well as the biofilm formation due to the metal ion release [22,[24], [25], [26], [27], [28], [29], [30], [31]]. Recently, researches on antibacterial metal alloys also indicated that Cu- and Ag-containing metal alloys also show anti-adhesion and anti-biofilm function due to the metal ion release [32,33].
3. Advantages and disadvantage of antibacterial metals and alloys
Antibacterial surface modifications on metal could provide strong antibacterial ability but also good biocompatibility, including but not limited to the following measurements:
-
1)
Antibacterial metal-implanted surfaces, including silver-containing (particle or ion) [[34], [35], [36], [37]] and Cu-containing [[38], [39], [40]] or Zn-containing [[41], [42], [43]] antibacterial surface.
-
2)
Nanoscale surfaces, including Ag-containing nanoparticle (NPs) [44], Cu-containing NPs [45] or Zn-containing NPs [[46], [47], [48]], gold-containing [49] surfaces due to the strong antibacterial property of metal nanoparticle (NP), even if confronts against drug-resistant bacteria [50,51].
-
3)
UV-activatable or photo-sensitive antibacterial surfaces. Ti2O coating becomes bactericidal under near ultraviolet light. It was reported that these surfaces require up to 80 min of UV exposure to eliminate 75–95% of bacteria [4,52]. However, it is unclear if the technology would be effective on osseointegrated implants or in deep peri-implant pockets due to limitations in the penetration of light. Recently, it has been discovered that TiO2 nanostructures could produce superior antibacterial activity in vitro and in vivo under the irradiation of 808 nm near-infrared (NIR) light that human body can withstand for a short time [53]. Wu [[54], [55], [56]] reported some novel coatings, such as red phosphorus/IR780/arginine-glycine-aspartic acid-cysteine, polydopamine/IR780@MnO2 and red phosphorus/graphene oxide film on titanium implants utilizing NIR light, exhibiting excellent antibacterial property. Meanwhile, other photo-sensitive materials, such as hydrogels [57] and chitosan/Ag/MoS2 [58], have also used to prepare antibacterial surfaces.
-
4)
Drug-loaded surfaces. The drugs include conventional antibiotics such as amoxicillin [59], vancomycin [[60], [61], [62]], gentamicin [63], tetracycline [64], minocycline [59,65], cephalotin [66] and chlorhexidine [59,67].
-
5)
Antibacterial peptide surfaces (AMPs). Compared with antibiotics, AMPs have drawn increasing attention as novel antibacterial agents to combat bacterial invasion due to their special mechanisms of action, non-inducible bacterial resistance and lack of detrimental effects on humans [[68], [69], [70]].
However, on the other hand, there are some potential disadvantages in the antibacterial surface modification:
-
1)
The risk of bacterial resistance and cell toxicity.
-
2)
Finite duration of antimicrobial activity.
-
3)
Coating delaminating on implants.
In comparison with the antibacterial coatings, the antibacterial metals and alloys are of following advantages:
-
1)
Long-term antibacterial ability. The whole alloy is of antibacterial activity, thus the antibacterial ability will not be lost due to the machining, implantation, wear or abrasion, corrosion, etc. On the contrary, wear, abrasion or machining will produce a fresh surface, which in turn enhances the antibacterial activity. But for antibacterial coating, including Ag- and Cu-containing coating, wear or abrasion will destroy the coating and significantly reduce the antibacterial efficiency.
-
2)
Easy control and easy preparation. Normal metal and alloy processing, including casting, metal forming and powder metallurgy, etc. can be used to prepare and produce antibacterial metals and alloys with different shapes including bar, sheet and even complex structure. The metal and alloy can also be machined to produce implants with complex structure without reduction of antibacterial properties. Normal sterilization treatments also can be used on the antibacterial metal and alloy devices without reduction of antibacterial properties.
-
3)
No potential drug-resistance. The antibacterial ability of the metals and alloys is mainly attributed the antibacterial mechanism of alloying element, such as Ag, Cu and Zn elements which have been proven to have broad spectrum antibacterial ability to nearly all bacteria and no drug-resistance has been reported so far. More recently, it was demonstrated that the antibacterial properties were mainly controlled by the precipitation of Ag-or Cu-containing phase rather than the Ag or Cu ion release [71].
However, there are also some drawbacks of antibacterial metals and alloys:
-
1)
Mechanical properties and corrosion property. Ag and Cu are not main alloying elements for antibacterial stainless steel, titanium alloy and magnesium alloy. The addition of these antibacterial elements will change the microstructure characteristic, and thus change the mechanical properties potentially as well as corrosion resistance. For example, Cu element is considered as one of impurity elements for magnesium alloy, which can significantly deteriorate the corrosion resistance or accelerate the degradation of magnesium alloy [72]. Optimization of the mechanical property, the corrosion resistance and the antibacterial ability of antibacterial alloys will be a big challenge. Especially for long-term application, the effect of the antibacterial alloying elements on the fatigue strength will be significantly important.
-
2)
Potential toxicity of the metal ion release. It has been widely accepted that antibacterial ability of metals and alloys comes mainly from the released metal ions, including Ag+, Cu2+ and Zn2+. In order to obtain high antibacterial activity, metal ion release concentration has to be high enough, which might cause local toxicity and sometimes accumulation in distant target organs. Thus, it is always critical to find out a correct balance between bactericidal effects and biocompatibility properties, especially as far as cytotoxicity, cytocompatibility and immunocompatibility are concerned. However, it has been shown recently that titanium alloys with low Ag ion and Cu ion release also exhibited strong antibacterial ability, which reduces the toxicity of metal ions.
-
3)
Function of surface modification. For biomedical application, the metal surface is normally modified by surface coatings technology, such as bone morphogenetic protein (BMP) containing coating, to improve the surface cell bioactivity. Some surface coatings separate the bacteria from antibacterial metal alloy, and reduce or eliminate the antibacterial property as a result. However, sand blasting, sandblasting and acid etch (SLA), alkaline and heat treatment and micro-arc oxidation (MAO) have been proven to have no influence on the antibacterial activity of antibacterial titanium [[73], [74], [75], [76]].
4. Selection of antibacterial alloying element
Antibacterial agents can be broadly classified into three major categories: organic, inorganic and natural. To develop antibacterial metal alloys, the selection of antibacterial alloying elements is very important. So far, it is widely accepted that Ag and Cu are normally alloying elements in the development of antibacterial metal alloys, in which Ag has been described as one of the earliest materials to be intentionally used in surgery for its bactericidal properties [77]. Actually, other metals also exhibit antibacterial property to some extent.
Sreekumari et al. [78] reported the ability of different metals to resist bacterial attachment, including Zn, Ni, Pb, Co, Mo, Zr, Cu, Sn and Ti. Except Sn and Ti, all other metals showed profound antibacterial property against E. coli and S. aureus by reducing the bacteria colony forming units (CFU) on their surface to less than 101 from 106 within a period of 24 h. It was also reported that Pb followed by Co and Cu were most effective metals those resisted bacterial attachment and growth by causing mortality [78].
Kawakami [79] assessed the antibacterial property of 21 metallic elements against E. coli and S. aureus by a plate counting method. Besides Ag, Cu and Zn, other elements, including Al, Co, Ni, Mo, Pd and W also exhibited antimicrobial activity (R > 2.0) against both E. coli and S. aureus, and Pt and Pb only against E. coli, and V and Zr only against S. aureus. In addition, the bactericidal efficiency (K value) of Ag and Cu was significantly higher than the values of other metals, indicating strong biocidal activities of Ag and Cu.
Miyano [80] also evaluated the antibacterial ability of some pure metals by two different methods: plate count method and shaking flask method (JIS Z2802:2000). The plate count method results after 24 h incubation indicated that pure Co, Ni, Cu, Zn, Zr, Mo and Pb exhibited strong antibacterial ability (more than 2log10 reduction in total viable cell, cfu/mL) against S. aureus and Ti, Co, Ni, Cu, Zn, Zr, Mo and Pb showed strong antibacterial ability (more than 2log10 reduction in total viable cell, cfu/ml) against E. coli. On the contrary, the shaking flask test after 24 h incubation indicated that only Pb showed strong antibacterial ability against S. aureus and Zn and Co against E. coli. In addition, their plate count results indicated the antibacterial efficiency was in the following order from high to low: Pb > Cu > Co > Zn > Ni > Zr > Mo.
Heidenau et al. [81] evaluated the antibacterial ability of several metal ions against S. epidermis by the plate count method. Their results confirmed that Hg+, Ag+ and Cu2+ exhibited very strong antibacterial property but Co2+ and Zn2+ exhibited no significant antibacterial effect at the investigated concentrations. Table 1 summaries the minimum inhibitory concentration (MIC) of several metal ions for different bacteria. Significant difference was found in MIC among different metal ions and for different bacteria. For example, MIC value of Cu ion for S. Typhimurium S9 was as high as 14000 μM while the value for P. phosphoreum was just 31.2 μM. Different researchers reported different MIC values due to the different testing methods.
Table 1.
Minimum inhibitory concentration (MIC) for different bacteria.
| Elements | MIC (μM) | Bacterium | Ref. |
|---|---|---|---|
| Ag+ | 37.4 | E. coli | [82] |
| 37.4 | S. choleraesuis | ||
| 74.7 | S. aureus | ||
| 40 | S. epidermis | [81] | |
| Co2+ | 240 * | S. epidermis | [81] |
| >508.5 | P. phosphoreum | [83] | |
| Cr3+ | 384 | P. phosphoreum | [83] |
| Cu2+ | 10000 | S. Enteritidis SE | [84] |
| 12000 |
S. Typhimurium S19; S. Typhimurium S20 |
||
| 14000 | S. Typhimurium S9 | ||
| 7000 | S. aureus | [82] | |
| 4000 | E. coli | ||
| 4000 | S. choleraesuis | ||
| 31.2 | P. phosphoreum | [83] | |
| 110 | S. epidermis | [81] | |
| Fe3+ | 0.89 | P. phosphoreum | [83] |
| Mn2+ | 26812.3 | E. coli; S. choleraesuis | [82] |
| 29143.9 | S. aureus | ||
| Ni2+ | >6779 | P. phosphoreum | [83] |
| Zn2+ | 11743 | S. aureus; E. coli; S. choleraesuis | [82] |
| >45.8 | P. phosphoreum | [83] | |
| >300 | S. epidermis | [81] | |
| <139.5 | S. aureus | [85] | |
| 155 | S. aureus | [86] |
Note: MIC data have been transferred into μM for comparison purpose.
Metal ion provides strong antibacterial ability, it on the other hand might lead to cytotoxicity or poor cytocompatibility. Heidenau et al. [81] evaluated cell toxicity of several metal ions by a growth inhibition test with L929 cell line. Their results showed that Ag+, Zn2+ and Hg2+ ions exhibited very strong cytotoxicity at low concentrations. Co2+ showed intermediate cytotoxicity whereas tissue cells tolerated relatively high concentrations of Cu2+ and Al3+. Table 2 summaries the LD50 value (a value which causes 50% cell death) of several metal ions to different cells. Although large difference in LD50 value was found, even for same cell line, Ag+, Cu2+, Co2+, Zn2+ and Ni2+ showed low LD50 value while Ti4+, Ta5+, Fe2+ and Fe3+ showed high LD50 value, indicating that Ag+, Cu2+, Co2+, Zn2+ and Ni2+ show potential higher cell toxicity than the others.
Table 2.
Cell toxicity of metal ions (LD50).
| Metal ion | Medium | Test method | LD50(μM) | Cell line | Ref. |
|---|---|---|---|---|---|
| Ag+ | DMEM with 10% FBS | MTT | 11.0 | human osteosarcoma cell line MG-63 | [87] |
| DMEM with 10% FBS | MTT | 9.0 | tumour-derived mouse macrophage cell line | ||
| L929: MEM with 10 vol% FBS | 10 vol% Giemsa staining | 4.25 | L929 | [88] | |
| MC3T3-E1: α-MEM with 10 vol% FBS | 2.77 | MC3T3-E1 | |||
| RPMI 1640 with 10 vol% FCS | WST-1 test assay | 3.5 | L929 | [81] | |
| Co2+ | Same as [88]above | 10 vol% Giemsa staining | 11.2 | MC3T3-E1 | [88] |
| 81.2 | L929 | ||||
| RPMI 1640 with 10 vol% FCS | WST-1 test assay | 34 | L929 | [81] | |
| Cr3+ | Same as [88]above | 10 vol% Giemsa staining | 12.7 | MC3T3-E1 | [88] |
| 743 | L929 | ||||
| Cu2+ | Same as [88]above | 10 vol% Giemsa staining | 15.9 | MC3T3-E1 | [88] |
| 41.5 | L929 | ||||
| RPMI 1640 with 10 vol% FCS | WST-1 test assay | 230 | L929 | [81] | |
| Fe3+ | Same as [88]above | 10 vol% Giemsa staining | 328 | MC3T3-E1 | [88] |
| 5420 | L929 | ||||
| Fe2+ | Same as [88]above | 10 vol% Giemsa staining | 583 | MC3T3-E1 | [88] |
| 6950 | L929 | ||||
| Ni2+ | Same as [88]above | 10 vol% Giemsa staining | 52.2 | MC3T3-E1 | [88] |
| 106 | L929 | ||||
| Ta5+ | Same as [88]above | 10 vol% Giemsa staining | 2060 | MC3T3-E1 | [88] |
| 1880 | L929 | ||||
| Ti4+ | Same as [88]above | 10 vol% Giemsa staining | 871 | MC3T3-E1 | [88] |
| 1090 | L929 | ||||
| Zn2+ | Same as [88]above | 10 vol% Giemsa staining | 90 | MC3T3-E1 | [88] |
| 92.8 | L929 | ||||
| RPMI 1640 with 10 vol% FCS | WST-1 test assay | 3.6 | L929 | [81] | |
| serum-free medium | MTT | 300 | Primary human aortic endothelial cells | [89] | |
| serum-free medium | MTT | 280 | Primary human aortic smooth muscle cells | [89] | |
| complete cell-culture medium | MTT | 215 | Primary human endometrial epithelial cells | [90] | |
| serum-free medium: 2 h + medium with serum: 24 h | MTT | 185 | Neuronal PC12 cells | [91] |
Note: a) MEM: minimum essential medium, b) DMEM: Dulbecco's modified Eagle's medium, c) FBS: fetal bovine serum, d) FCS: fetal calf serum, e) RPMI: Roswell Park Memorial Institute-a cell culture medium.
Metal ion provides strong antibacterial ability, it on the other hand might lead to cytotoxicity or poor compatibility. Heidenau et al. [81] evaluated cell toxicity of several metal ions by a growth inhibition test with L929 cell line. Their results showed that Ag+, Zn2+ and Hg2+ ions exhibited very strong cytotoxicity at low concentrations. Co2+ showed intermediate cytotoxicity whereas tissue cells tolerated relatively high concentrations of Cu2+ and Al3+. Table 2 summaries the LD50 value (a value which causes 50% cell death) of several metal ions to different cells. Although large difference in LD50 value was found, even for same cell line, Ag+, Cu2+, Co2+, Zn2+ and Ni2+ showed low LD50 value while Ti4+, Ta5+, Fe2+ and Fe3+ showed high LD50 value, indicating that Ag+, Cu2+, Co2+, Zn2+ and Ni2+ show potential higher cell toxicity than the others.
According to the general antibacterial mechanism, the strong antibacterial ability of metal is due to the accumulation of metal ion, thus high metal ion concentration will result in high antibacterial property. But on the other hand, the high metal ion concentration will also lead to cell toxicity. With above considerations, the alloying elements with low MIC and high LD50 will be the best choice for developing antibacterial metal alloys, such as Fe3+, Ag2+, Co2+, Cu2+ and Zn2+. Ag2+, Cu2+ and Zn2+ have been widely used as antibacterial agent but Fe3+ and Co2+ have not yet been reported to be an antibacterial agent.
4.1. Ag
Silver has a strong inhibitory and bactericidal effect on a range of bacteria, fungi and viral pathogens [92]. Silver ions and silver-based compounds are highly toxic to microorganisms [93] showing strong biocidal effects on as many as 16 species of bacteria [94]. The antibacterial activity of silver was first demonstrated in the 19th century. Now, the use of various forms of silver as a topical agent is commonplace [92,95]. Silver is certainly the metal most commonly used with the purpose to confer anti-infective properties to biomedical devices for its oligo dynamic antibacterial activity, i.e. exhibiting bactericidal/bacteriostatic activity at very low concentration [12] for dental and orthopedic implant applications [96,97]. Widely documented use of silver is prophylactic treatment of wounds, water disinfection, pleurodesis, cauterization etc. Silver was used as a biocide to sterilize recycled drinking water aboard of the NASA space shuttle and MIR space station [98]. However, the use of silver as bulk material in medical devices has progressively been ceasing. On the other hand, the utilization of Ag element in antibacterial surface modification has progressively been flourishing, such as nanoparticles loaded thin film [99,100], doped solid coatings [101], hydrogel materials [102], micro- and nanoparticles reinforced antibacterial glasses [103] and antibacterial polymer material [50]. Meanwhile, Ag element has also been used as an alloying element in preparation of antibacterial metal alloys [104,105].
It was reported that silver at low concentrations was not cytotoxic for osteoblast in vitro [106]. Controversially, debate still persists over the possible inactivation of silver mediated antibacterial activity in physiological fluids and over the low biocompatibility index of silver determined by the low threshold concentration for cytotoxic effects, especially inform of nanoparticles. Li [107] showed that nano-Ag had the potential to induce embryo cytotoxicity, although the toxicity was lower than Ag+ ion.
4.2. Co
Although some researchers reported that Co metal exhibited strong antibacterial ability, as mentioned above, Co2+ showed a high MIC value to several bacteria as listed in Table 1, and low LD50 value to cells as listed in Table 2, which indicates that Co and Co2+ are not a proper antibacterial alloying element. So far, Co or Co2+ has not been used as an alloying element or antibacterial agent in the development of antibacterial materials and coating.
4.3. Ce
Recently, Jing [108] reported for the first time that Ce-containing stainless steels with 0.11 wt% Ce and 3.25 wt% exhibited very strong antibacterial ability (>99% reduction). In comparison with antibacterial agent Cu and Ag elements, Ce element showed a high antibacterial efficiency, only 0.11 wt% Ce in steel provided with strong antibacterial properties. As for the antibacterial mechanism, it was suggested that Ce ion released from the Ce-containing alloy might have similar antibacterial effects as Cu ion and Ag ion. However, Ce release concentration was not detected in their report.
4.4. Cu
Long time ago, copper was used to clean up wounds on the chest and to purify water, to treat local skin ulcers in the legs and skin diseases, syphilis and tuberculosis. After copper was certified by the US Environmental Protection Agency (EPA) as having antibacterial properties in 2008 [109], its potential application for antibacterial activity has aroused more and more commercial interest. It has been reported that a copper surface can complete kill MRSA and E. coli within a few hours [110,111]. Many researchers have studied the antibacterial and bactericidal concentrations of Cu ions against different types of bacteria. Copper-sensitive bacteria, e.g., K. aerogenes were inhibited in their growth and survival in a range of 10−8-10−6 M Cu2+ ion concentrations [112]. When the Cu2+ concentration was 10−5 M, the sterilization rate to S. aureus was 92% while the sterilization rate to E. coli was 93% at 5 × 10−6 M Cu2+ [[113], [114], [115]]. Recently, Cu has been widely used to develop antibacterial materials, and some reviews have been published, including Cu-containing ceramic [116,117], Cu-containing polymer composites [118], and Cu-containing metal alloys [119,120].
In addition, copper is an essential metal ion to the proper functioning of organs and metabolic processes, which is incorporated into a variety of proteins and enzymes of organisms, such as cytochrome oxidase, superoxide dismutase, internal plasma copper blue protein, dopamine, β-hydroxylase, etc. [121]. Cu deficiency is harmful for human health, which may not only result in anemia, cardiac hypertrophy, coronary heart disease, arthritis, and osteoporosis, etc., but also influence the human secretion system and immune function, especially cardiovascular system [122]. The World Health Organization (WHO) reports that the minimal intake to prevent copper deficiency is 0.9 mg/day [123]. Cu ion can promote osteoblast proliferation, differentiation and migration [[124], [125], [126], [127], [128], [129]]. Cu ion also plays an important role in the cardiovascular system and has beneficial biomedical functions in promoting endothelial cell growth, inhibiting excessive proliferation of arterial smooth muscle, and reducing thrombosis [120,[130], [131], [132], [133]]. Zhang [134] recently proposed that in-stent restenosis might be prevented by the constant release of Cu ion due to inhibition of migration and proliferation of vascular smooth muscle cells by Cu ion. Due to the biofunction of Cu ion, it is proposed that the Cu-containing materials, including metal alloys might have both biofunction and antibacterial activity by special alloy design.
However, high concentrations of Cu ions will induce an inhibition of growth and have a toxic effect on humans [16]. Many researchers reported that the toxicity of Cu depended on the existing form of Cu element. Thit [135] argued that all the three existing forms of Cu including poly-CuO, CuO NPs or Cu2+increased cell death and altered cell cycle progression, but poly-CuO caused the most severe effects. Bondarenko [136] indicated dissolution of CuO particles was the key factor triggering the reactive oxygen species (ROS) and DNA damage responses in bacteria. It was also reported that Cu ion might be related to Alzheimer disease [137].
4.5. Fe
Fe is a necessary trace element for human being, which is involved in many Fe-containing enzymes and proteins [138,139]. Fe plays significant roles in the human body, including the transport, storage and activation of molecular oxygen, and the reduction of ribonucleotides and dinitrogen [138]. WHO permissible maximum tolerable daily Fe intake is < 56 mg [140].
Wang's results [83] on the toxicity of some metal ions against P. phosphoreum indicated that Fe3+ showed the highest inhibition, followed by Cu2+ > Zn2+ > Co3+ > Cr3+ > Ni2+. It started to inhibit the bioluminescence at the concentration of 0.01 mg/L (0.18 μM) and reached complete inhibition when the concentration was only 0.05 mg/L (0.89 μM). However, pure Fe and Fe alloy do not exhibit any antibacterial properties.
So far, Fe or Fe ion has not been used as an alloying element or antibacterial agent in the development of antibacterial materials and coating. However, previous studies have revealed that pure Fe and Fe-based alloys were “safe” degradable implantable materials because they did not seem to be associated with inflammation, neointimal proliferation or thrombotic events [[141], [142], [143], [144]]. Moreover, Fe and Fe-based alloys do not have the issue of gas release during degradation compared with Mg and Mg-based alloys [145].
4.6. Ga
Recently, it was reported that gallium-doped specimens showed the best ALP synthesis and antibacterial properties [146]. In the report, Ga-doped coating was produced on titanium substrate by an anodic spark deposition (ASD) method. Ga-doped specimen reduced the bacterial viability by 70–80% against multi drug resistant (MDR) pathogen A. baumannii (DSM 30007) as well as two A. baumanni clinical isolates (AB1 and AB2). The antibacterial ability of Ga-doped specimen was much stronger than Ag-doped specimen.
Although it was reported that the presence of anatase formed during ASD process might confer antibacterial activity to all the ASD coatings, this effect was enhanced significantly by the presence of Ga. It was thought that the antimicrobial activity of the Ga-doped surface was mainly related to competition with iron in a “Trojan horse” mechanism: in bacterial metabolism, the role of Fe3+ ions is crucial, and is mediated by intra-membrane transport via siderophores; gallium ions (Ga3+) can effectively compete with Fe3+ for binding to siderophores, thus interrupting crucial Fe-dependent metabolic pathways in the bacterium. Moreover, Ga3+ can act intracellularly affecting iron homeostasis leading to different pathways damage.
4.7. La
It was reported addition of only 0.42 wt% La into 316L SS content could obtain excellent antibacterial activity (>99% reduction) against E. coli and S. aureus [147]. Another research also indicated that La- and Ce-containing 316L-4.36Cu alloy showed antibacterial activity to the sessile sulfate reducing bacteria (SRB) at the first 7 days due to the addition of rare elements La and Ce but the antibacterial activity disappeared at 21st day [148].
4.8. Sn
Many researchers reported the dopant Sn played a vital role in enhancing the antimicrobial activities [[149], [150], [151]]. Verissimo's research [149] on S. aureus growth on Ti–35Nb–3Sn showed for the first time that adding Sn to β-type Ti alloys strongly decreased the adhesion of S. aureus, but also increased mechanical properties. As for the antibacterial mechanism, it was proposed that the addition of Sn might change the wettability which may have changed surface properties to repel bacteria. It was also reported that the bacteria attachment decreased with the presence of Sn in the Ti oxide layer due to the change in the physical and chemical properties, such as wettability, surface architectures and chemical composition (SnO2). However, no negative sample was used in their studies. Research on the influence of Sn doping on the antibacterial activity of ZnO films found positive relationship between Sn doping level and antibacterial activity [151].
4.9. Sr
In recent years, strontium (Sr) has been incorporated into dental and orthopedic biomaterial to reduce microbial contamination and the antimicrobial activity of Sr2+ ions can be used to enhance the use of medical devices by inhibiting bacterial growth and reproduction and impeding permeability of cytoplasmic membrane, cell wall synthesis, replication of bacterial chromosomes and cell metabolism [[152], [153], [154]]. In addition, Sr is one of trace minerals (0.00044% of body mass) predominantly present in the calcified animal tissue. Shorr and Carter [155] discovered the usefulness of low doses of Sr in the treatment of osteoporosis in 1952. Several in vitro and in vivo studies on Sr-substituted hydroxyapatite (HA), biological glass and bio-ceramics revealed that they promoted osteoblast proliferation and down-regulated osteoclastogenesis in both normal and osteopenic cells, with an increase in the alkaline phosphatase activity.
Guida's research [156] suggested that the bactericidal action of strontium was more significant than that of fluoride. It was also suggested that the fast degradation of Sr-containing ceramic increased the local pH, which might inhibit the bacteria [157]. The antibacterial properties of HA nanoparticles against E. coli, S. aureus and Lactobacillus were reported to be improved after the calcium was half or totally substituted by strontium and the antibacterial ability was strongly dependent on the Sr concentration [158]. However, Sr2+ in a concentration of 0.19 mol/L to 1.11 mol/L did not exhibit any antibacterial function to A. viscosus, A. naeslundii, A. odontolyticus, S. mutans, S. salivarius, S. sorbinus, L. caesi, L. acidophilus, P. gingivalis, P. intermedia and A. actinomycetemcomitans [159]. It was also suggested that Sr release concentrations above 0.16 mmol/L (14 ppm) did not further improve bactericidal action [160].
Sr has not been used as an alloying element for titanium, steel and cobalt alloy design as biomedical materials, but has been used in magnesium alloys [[161], [162], [163], [164], [165], [166], [167], [168], [169]].
4.10. Zn
Zinc is used as an antibacterial agent in dental and formulated into oral health products to control plaque such us mouth rinses and toothpaste [170,171]. Based on this knowledge, Zn ion has been widely used in surface modification of metal implant as antibacterial agent to provide good antibacterial ability [[172], [173], [174], [175]].
However, the antibacterial mechanism of Zn containing coating has not been systematically elaborated [174]. Numerous studies have been conducted to clarify the antibacterial mechanism of ZnO, including some reviews [176,177], while the dispute remains existing. Antibacterial mechanism of ZnO is publicly recognized as ROS generation [178,179] and Zn2+ ion release [180], but most researchers maintain that the antibacterial property of ZnO mainly attributes to ROS generation. Li [178] considered ROS generation was the main antimicrobial factor for the photoexcited ZnO, because ROS concentration produced by nano-ZnO and their bacterial mortality rate kept linear relationship. Hu [179] observed no obviously high concentration of zinc ions when researched Zn-incorporated TiO2 coatings on titanium, thus it was believed the generation of ROS played a leading role in this work. In addition, for antibacterial mechanism of ZnO nanomaterials, increase membrane dysfunction [181], and internalization between the ZnO particles and the bacteria caused by electrostatic forces [182] are taken into account as well.
Compared with Ag and Cu, although Zn possesses a relatively weaker antibacterial activity [173], Zn ion shows non-cytotoxicity in a ranges from 10−6 M to10−5 M, as illustrated in Table 3. In addition, Zn plays important roles in DNA synthesis, enzyme activity, nucleic acid metabolism, biomineralization and hormonal activity, and it has long been recognized that zinc can stimulate bone formation [173,[183], [184], [185], [186]].
Table 3.
Zinc ion cytotoxicity.
| Concentration of Zn (μM) | Medium | Cell | Exposition time (h) | Test | Result | Ref |
|---|---|---|---|---|---|---|
| 20–80 | serum-free medium | Rat retinal cells | 24 | LDH |
20-40 μM: little cell death; 50 μM: 30%–40% cell death; 60-80 μM: almost 100% death |
[187] |
| 1–100 | serum-free medium | Human proximal tubular cells | 24–96 | MTT |
1-10 μM: ≥ 80% viability at 24–96 h; 50 μM: ≥ 80% viability until 48 h while then 30%–40% cell death; 100 μM: significant cell reduction at 24–96 h |
[188] |
| 2–300 | DMEM/MEM +5%FBS |
U–2OS (human cell line derived from osteosarcoma) | 24 | WST-1 |
2-120 μM: > 70% viability, 180–300 μM: nearly 100% cell death |
[189] |
| 2–300 | DMEM/MEM +5% FBS |
L929 | 24 | WST-1 |
2-80 μM: > 70% viability, 120-300 μM: nearly 100% cell death |
[189] |
Note: a) MEM: minimum essential medium, b) DMEM: Dulbecco's modified Eagle's medium, c) FBS: fetal bovine serum.
5. Standards or methods
So far, there is only one standard to assess the antibacterial activity of metals and alloys, SN/T 2399-2010 China, but there are several methods and standards to measure the antibacterial activity of plastic materials, as listed in Table 4. The standard SN/T 2399-2011 is based on JIS Z 2801. In all test methods, glass wear such as Petri dish or culture plate is normally selected as a negative sample. Also, a well-known metals and alloys that do not show antibacterial ability such as pure titanium is selected as a control sample.
Table 4.
Some Standards for the measurement of the antibacterial activity of solid materials.
| Country/Organization | Standards No. | Title | Remarks |
|---|---|---|---|
| China | SN/T 2399-2010 | Evaluation method for antibacterial metallic materials | Based on JIS Z 2801―2000 |
| ISO | ISO 22196:2016 | Measurement of antibacterial activity on plastics and other non-porous surfaces | ISO22196:2007 is Based on JIS Z 2801―2000 |
| China | GB/T 31402-2015 | Plastics-Measurement of antibacterial activity on plastics surfaces | ISO 22196:2007, IDT |
| Japan | JIS Z 2801―2000 | Antimicrobial products―Test for antimicrobial activity and efficacy | |
| UK | BS ISO 22196:2016 | Measurement of antibacterial activity on plastics and other non-porous surfaces | ISO 22196:2016 |
| USA | ASTM E3031-15 | Standard Test Method for Determination of Antibacterial Activity on Ceramic Surfaces. | Ref. ISO-22196 |
| USA | ASTM G21―15 | Standard Practice for Determining Resistance of Synthetic Polymeric Materials to Fungi | |
| ISO | ISO20645: 2004 | Text fabrics-Determination of antibacterial activity-Agar diffusion plate test | |
| China | GB/T 20944.1–2007 | Textiles—Evaluation for antibacterial activity—Part 1: Agar diffusion plate method | ISO 20645:2004 |
5.1. Agar diffusion plate test
Specimens of the materials to be tested are placed on two-layer agar plates. The lower layer consists of a culture medium free from bacteria and the upper layer is inoculated with the selected bacteria. The specimens are tested on both sides. The level of antibacterial activity is assessed by examining the extent of bacterial growth in the contact zone between the agar and the specimen and, if present, the extent of the inhibition zone around the specimen. Thus, the width of the inhibition zone represents the antibacterial ability: the larger the inhibition zone is and the strong the antibacterial activity is.
According to the Standard ISO 20645: 2004 (BS EN ISO 20645: 2004 or GB/T 20944.1), the hot agar solution is first spread evenly with a Conrage stick on the sterilized Petri dishes and is allowed to congeal under sterile conditions. A suspension of bacterium, such as S. aureus or E. coli, is sprayed evenly over the total area of each Petri dish. A test sample, a control sample and a positive sample are separately placed and contact with the agar closely in the Petri dish. After this, the Petri dishes with samples are incubated immediately at (37 ± 1) °C for 18 h–24 h. The antibacterial activity is accessed by the width of an inhibition zone (H) around the sample calculated by the following formula
| (1) |
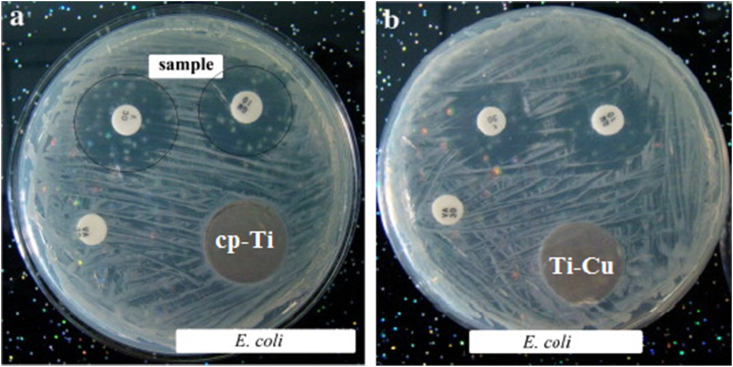
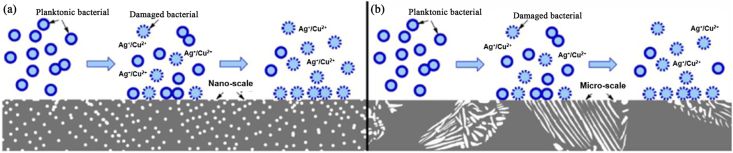
where, H is the inhibition zone in mm, D is the total diameter of sample and inhibition zone in mm and d is the diameter of the sample in mm, respectively. After measuring the inhibition zone, the specimens will be removed from the agar with a pair of tweezers and the bacterial growth in the nutrient medium under the specimen with a microscope at 20 times magnification. Inhibition zone up to 1 mm with no bacteria growth under specimen or no inhibition zone with no bacteria growth means good antibacterial effect of the specimen. As an example, Fig. 4 shows the inhibition zones around samples in the agar diffusion plate test. An inhibition zone was clearly observed around the white positive sample, indicating that the positive sample exhibited strong antibacterial ability against E. coli. On the other hand, there was no inhibition zone around cp-Ti and Ti–Cu alloy, indicating that both cp-Ti and Ti–Cu alloy did not kill the surrounding bacteria.
Fig. 4.
Inhibition zones around different samples against E. coli (the white sample is the positive sample). a) cp-Ti and b) Ti–Cu samples [18], copyright 2013, Elsevier B.V.
5.2. Plate-count method
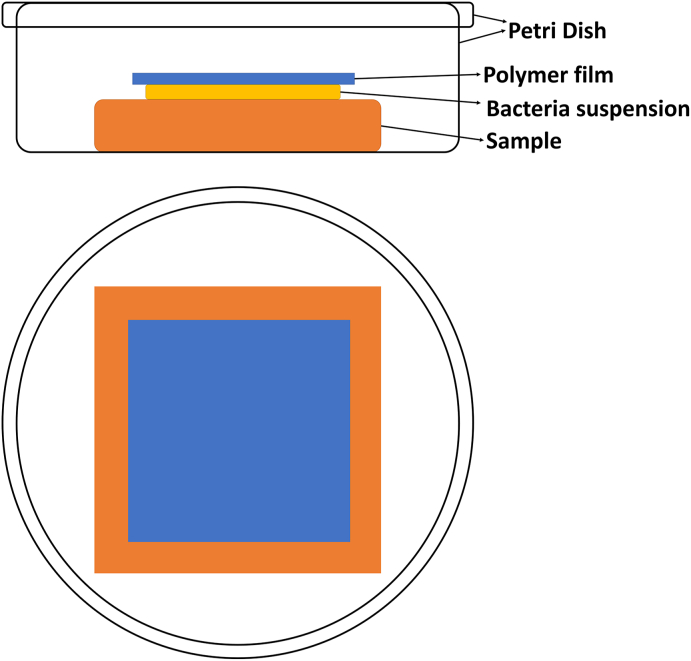
Although the plate-count method is intended to evaluate the antibacterial activity of antibacterial plastics (JIS Z 2801―2000, ISO 22196-2016, GB/T31402-2015) and other non-porous surface of products (ISO 22196-2016), this method has been widely adopted to assess the antibacterial activity of metals and alloys. The experiment process has been described in detail in JIS Z 2801―2000, ISO 22196 or GB/T31402-2015. Briefly, samples are placed in Petri dishes separately. Then, the bacterial suspension (0.4 mL in the standard, not less than 0.1 mL) is dripped onto the sample, including the test sample, the control sample and the negative sample. After this, the sample is covered by a sterile polyethylene film with a dimension to make sure that the suspension spreads to, but does not leak beyond the edges of the film, as shown in Fig. 5. Then the samples with bacterium are incubated at (35 ± 1) °C for 24 h under a humidity of ≥90%. After the incubation, 10 mL or more sterilized physiological saline solution is added to the Petri dish to wash the sample and the polymer film completely to make sure that no bacterium is left on the sample and the film. Then 0.1 mL the above washing solution is inoculated onto nutrient agar plates and incubated at (35 ± 1) °C for 24 h under a humidity of ≥90%. The active bacteria colonies are counted. Three samples are assessed for each type of samples. The antibacterial activity, R, is calculated by the following formula:
| (2) |
where, Ncontrol and Nsample are the average numbers of the bacterial colony on the control sample and the tested sample, respectively.
Fig. 5.
Schematic diagram of the plate-count method.
5.3. Live/dead stain
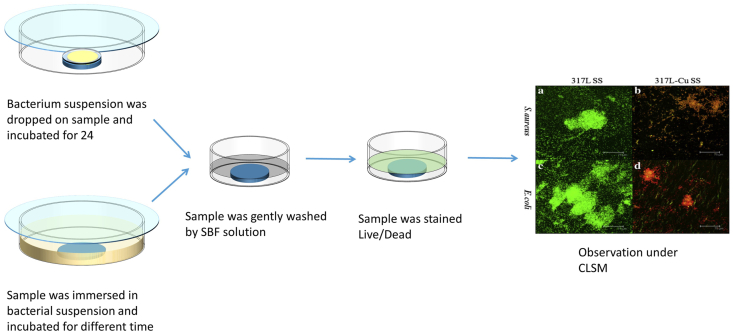
Live/dead stain is another method which has been widely used to observe the bacterium adhesion, biofilm and dead bacterium in order to assess the antibacterial properties. In this method, as shown in Fig. 6, bacterial suspension is dripped onto the tested samples or the sample is immersed in a bacterium suspension, and then incubated at 37 °C for 24 h. After incubation, the sample surface is washed gently for three times by using phosphate buffer saline (PBS) to remove the traces of culture medium. Then, two fluorescent dyes, LIVE/DEAD® BacLight™ Bacterial Viability Kits L7012 containing SYTO-9 dye and PI dye are used to stain the bacteria on the sample surface according to the instruction in dark at room temperature. After that, the dyed sample is observed under a confocal Laser scanning microscope (CLSM) at 514/488 nm in an argon laser. The bacteria with green color mean living bacteria and the ones with red color are dead bacteria. The inserted figures in Fig. 6 shows S. aureus and E. coli on 317L stainless steel and 317L-Cu stainless steel after incubation at 37 °C for 24 h [190]. Most of the bacteria on the surface of 317L stainless steel were stained in green whereas bacteria on the surface of 317L-Cu stainless steel were stained in both red and green, and the red color was significantly dominated.
Fig. 6.
Live/Dead stain method used to observe the surface bacterium. The inserted Fluorescent images show S. aureus and E. coli on surfaces of 317L stainless steel and 317L-Cu stainless steel after incubation at 37 °C for 24 h [190], copyright 2012, Elsevier B.V.
5.4. Immersion test
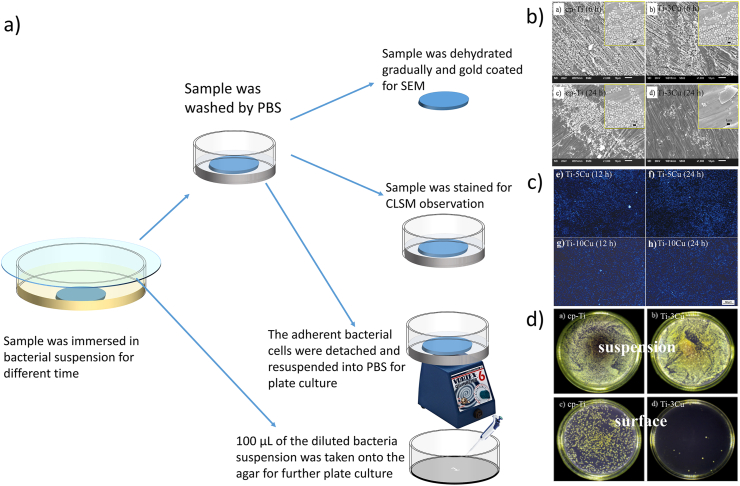
In some cases, the antibacterial activity against both the adhered bacteria and the planktonic bacteria properties need to be investigated, thus the immersion test method will be used. In the immersion test, as shown schematically in Fig. 7, the sample is immersed in a bacterium suspension, and incubated at 37 °C for 24 h (or for different time to assess the dynamic antibacterial properties), then the sample is taken out and washed gently by sterilized SBF solution to remove non-adherent bacterial cells. After this, the sample is gradually dehydrated and gold coated for SEM evaluation, as shown in Fig. 7b or the sample is stained with biological stain (400 μL Triton X-100 and 0.4 μL DAPI solution for example) for fluorescence microscope observation, as shown in Fig. 7c, or the sample is Live/dead stained as described above. By this way, the bacterium adhesion or biofilm formation can be clearly observed, which can directly reflect the anti-adhesion and anti-biofilm property of metal and alloy. Sometimes, the adherent bacteria cells on the sample are detached and resuspended into PBS by a two-step vortexing procedure at 4 °C for 1 min for each step to avoid unnecessary cell deaths. Serial dilutions (10−2 and 10−3) of the suspensions are plated on BHI agar plates and incubated overnight, followed by visual counting of colony forming units (CFUs) [191], as schematically shown in Fig. 7d. The antibacterial activity can be calculated by Eq. (2).
Fig. 7.
(a) Schematic diagram of the immersion method. (b) cell adhesion and biofilm under SEM [193], copyright 2018, Elsevier B.V.; (c) DAPI staining P. gingivalis on cp-Ti and Ti–10Cu alloy [194], copyright 2016, The Japanese Society for Dental Materials and Devices; (d) typical bacterium colonies from sample surface and the suspension [193], copyright 2018, Elsevier B.V.
Meanwhile, 100 μL of the bacteria suspension is taken onto the agar for further plate culture at 37 °C for 24 h and the bacteria colony is counted to assess the antibacterial properties of the sample against the planktonic bacteria by Eq. (2) [192], as shown in Fig. 7d.
5.5. In vivo test
In fact, antibacterial metal implants are in continuous contact with physiological fluids, including plasma in the human body and saliva in the oral cavity. Thus, in vivo microbial adhesion and plaque formation on biomaterial surfaces is influenced by factors such as multi-species oral pathogens, “host adhesions” (e.g. fibrinogen, fibronectin, collagen, and plasma albumin), host cells (cells of the innate and adaptive immune system) and the pellicle, a rapidly created conditioning film which covers a newly implanted device [2]. Another important limitation of in vitro studies is the protein concentration in artificial body fluids. This is because simply using a single host protein or a small selection of these cannot reflect the highly complex oral conditions in vivo [2].
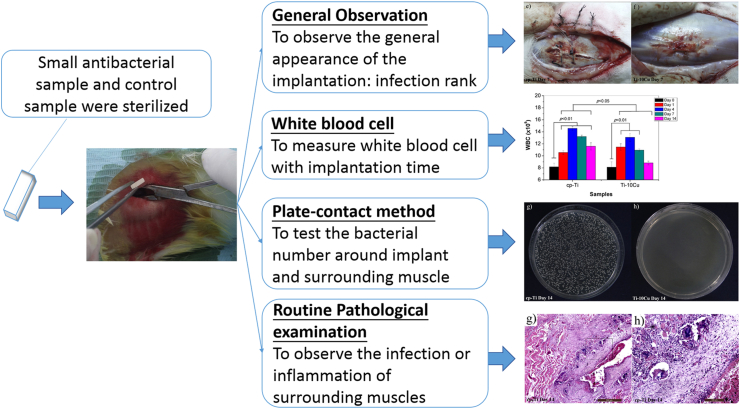
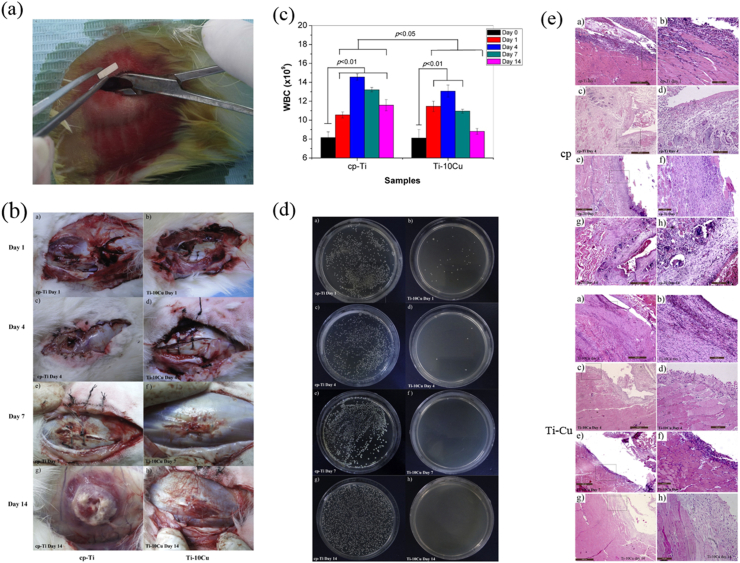
However, no standard on the in vivo test method has been set up so far. Recently, an in vivo test was used to determine the in vivo antibacterial activity of Ti–Cu alloy [195]. Briefly, sample with bacterium was implanted in the muscle of a rabbit. Postoperatively, the general observation, the white blood cell test, the plate-count method and the routine pathological examination were conducted to examine the inflection or inflammation situation surrounding the implantation site to reveal the in vivo antibacterial activity, as illustrated in Fig. 8.
Fig. 8.
Illustration of in vivo test of antibacterial titanium alloy. The inserted images show the general appearance, the blood cell test, the typical bacterium colonies and the pathological examination in vivo [195], copyright 2019, Elsevier B.V.
6. Antibacterial stainless steels
Stainless steels have been widely used as a biomaterial for fabricating cardiovascular stents/valves, orthopedic prosthesis, dentistry and other devices and implants used in biomedicine due to their good mechanical properties, corrosion resistance, biocompatibility and low price [196]. However, the lack of antibacterial properties is their great weakness in biomedical application. Nisshin Steel (Tokyo, Japan) developed the first copper-containing antibacterial stainless steel in the late 1990s [197,198]. Since the beginning of this century, lots of researches have been carried out and various types of copper-containing or silver-containing antibacterial stainless steel have been investigated so far including ferritic antibacterial stainless steel, austenitic antibacterial stainless steel, martensitic antibacterial stainless steel, as well as duplex antibacterial stainless steels [199,200]. Antibacterial alloying elements such as Cu and Ag are added into traditional stainless steel, which provides traditional stainless steel with antibacterial function and therefore is expected to provide a new approach to solve the problem of peri-implantitis for biomedical application [16].
6.1. Antibacterial properties
There are lots of factors that have influence on the antibacterial properties of stainless steel. As listed in Table 5, Cu-containing stainless steels exhibit excellent broad-spectrum antibacterial properties against lots of bacteria, such as S. aureus, B. coil, C. albicans, S. epidermis, S. lutea, E. coli, S. marcescens, B. pumilus, E. faecalis, S. fexneri, P. aeruginosa, P. sp as well as P. gingivalis, etc. However, antibacterial stainless steel shows different resistant ability to different bacteria. Nan [201,202] reported that antibacterial stainless steel had relatively stronger effect to Gram-negative bacteria than to Gram-positive bacteria, possibly because Gram-negative bacteria possessed thinner cell wall and were more susceptible to be damaged. The antibacterial stainless steel currently developed is mainly targeted at E. coli and S. aureus which are most common causes of implant-associated infections.
Table 5.
Antibacterial stainless steels.
| Alloy system | Alloy | Bacteria (antibacterial rate[Ref.]) |
|---|---|---|
| Ferritic stainless steel | 430-1.8Cu |
S. aureus, E. coli, B. coil, C. albicans, S. epidermis, S. lutea, S. fexneri and P. aeruginosa (all > 99% [199,201]); B. subilis (80% [199]); S. marcescens, B. pumilus, E. faecalis (98.2%, 97.7%, 91.0%, respectively [201]) |
| Austenitic stainless steel | 304-xCu (x = 1.5–5.5, 9,18 wt%) |
S. aureus (23.4%–99.9% [199,203,204]); E. coli (20%–99.9% [[204], [205], [206], [207]]); B. coil (10.6%–99% [200]); C. albicans, S. epidermis, S. lutea, S. fexneriand P. aeruginosa (all ≥ 99% [199,201]); P. gingivalis (100% after 12 h [16]); B. subilis(80% [199]); S. marcescens, B. pumilus, E. faecalis (99.5%, 92.4%, 93.6%, respectively [201]); planktonic bacteria in Tap Water (33.3%, 96%, 76.7% at 24 h, 48 h, 72 h [208]) |
| 304-xAg (x = 0.039–0.3 wt%) | E. coli (70.8–99.9% [209]); S. aureus (99.7%–99.9% [209]); P. sp [210] | |
| 304-xCe (x = 0.01–3.25 wt%) | E. coli (12.3–99.0% [108]); S. aureus (74.0%–99.4% [108]) | |
| 316-xCu (x = 2.46–4.5 wt%) |
S. aureus (95.2%–99.9% [211,212]); E. coli (25%–99% [[211], [212], [213]]); S. epidermidis (94.1% [212]) |
|
| 316L-xLa (x = 0.05–0.42 wt%) | S. aureus (15%–99.6% [147]); E. coli (9%–99.9% [147]) | |
| 317-4.5Cu |
E. coli (99.9% in vitro and in vivo [214], 92.7% in the artificial urine for 24 h and 90.3% in the human urine for 6 h [215]); S. aureus (99.0% [32,214,216], in vivo [214]) |
|
| Martensitic stainless steel | 410-xCu (x = 1.52–4.74 wt%) | E. coli (96–99.99% [217]) |
| 420-xCu (x = 0.4–5 wt%) |
S. aureus (35.4%–99.9% [197,198,[218], [219], [220]]); E. coli (43.6%–99.9% [218]) |
|
| Cr17Ni4–Cu (x = 3.4–5.06 wt%) | S. aureus (82%–99% [221]) | |
| Duplex stainless steels | 200-xCu (x = 1.45–3.57 wt%) |
S. aureus (42.8%–93.6% [202,222]); E. coli (56.1%–97.1% [202,222]) |
| 2205-0.2Ag | E. coli (100% [223]), S. aureus (99.6% [223]) | |
| 2205–3.02Cu | P. aeruginosa (33.1% [224], 99.5% [225]) | |
| Cr26Ni5Mo2-3.17Cu-0.07Ag | E. coli (>99% at 3 h and 6 h [226]) |
Note: 24 h incubation as default.
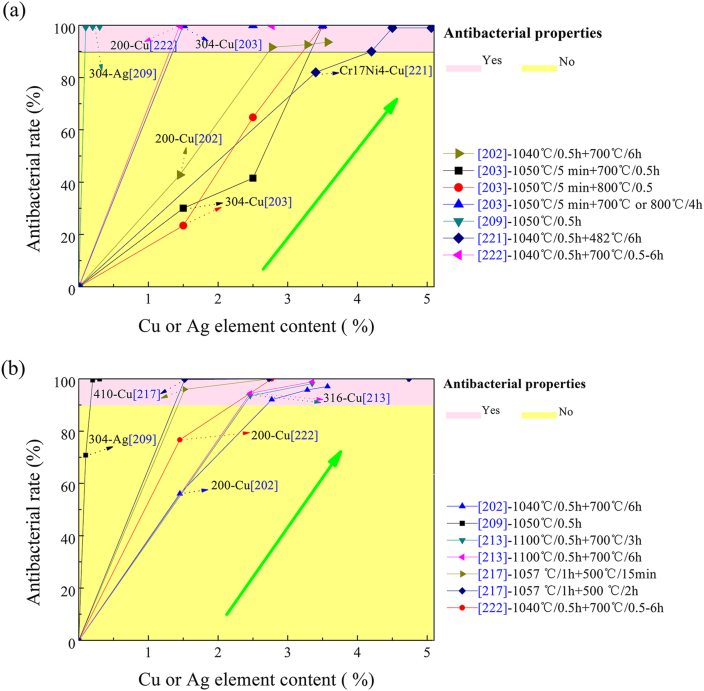
The content of antibacterial alloying element in stainless steels plays a very important role in the antibacterial properties. Normally, high content leads to strong antibacterial ability. However, high Cu content for example on the other hand might has side effect on the mechanical properties, the processing parameter and the corrosion resistance. Cu content in stainless steels is normally in a range of 1.52–5.5 wt% to obtain strong antibacterial ability. Fig. 9 summarizes the effect of Cu or Ag content on the antibacterial rate of some antibacterial stainless steels. 304 stainless steel containing 1.5–2.5 wt% Cu did not exhibit good antibacterial ability (<65%) in aging state, but increasing the Cu content to 3.5 wt% improved the antibacterial rate greatly to as high as > 99.99% [203]. Nan [222] reported that 200 stainless steel with 1.45 wt% Cu showed only 78.7% reduction against E. coli and >99.99% against S. aureus, but 200 stainless steel with 2.77 wt% Cu showed strong antibacterial activity (>99.99%) against both E. coli and S. aureus, indicating Cu content in 200 stainless steel has to be higher than a critical value, such as 2.77% in their study, to get good antibacterial ability. Wang [221] also revealed that the Cu content in a Cr17Ni4-(3.4–5.06)Cu had to be 4.2 wt% in order to get good antibacterial activity (>90%) or 4.5% Cu to obtain strong antibacterial ability (>99.9%).
Fig. 9.
Relationship between Cu content and antibacterial rate of stainless steel. a) against S. aureus (data from Refs. [202,203,209,221,222]) b) against E. coli (data from Refs. [202,209,213,217,222]).
Ag element has much stronger antibacterial ability than Cu, thus the Ag content in stainless steel is significantly lower than Cu content, about 0.039–0.3 wt% Ag. 2205-0.2Ag double-phase stainless steel exhibited an excellent bacteria-inhibiting effect on E. coli and S. aureus with an antibacterial rate of 100% and 99.5%, respectively [223]. Liao [209] found 304 austenitic stainless steel with 0.2 wt% Ag had an excellent antibacterial rate above 99%. With the consideration of reducing corrosion resistance due to the Cu-rich precipitate, small amount of Ag was also added to a Cu-containing dual-phases stainless steel to prepare an antibacterial dual-phase stainless steel. The alloy exhibited strong antibacterial ability (>99.4% reduction) against E. coli at 3 h incubation [226].
It was also reported the more the Ce content in the stainless steel was, the stronger the antibacterial ability of the steels appeared and 304 stainless steel with merely 0.11% Ce (304–0.11Ce) has strong antibacterial activity (98.9% reduction against E. coli and 99.1% against S. aureus) [108]. The addition of La also contributed to the antibacterial activity in a dose dependent method [147]. When the La content in 316L was about 0.05%, no antibacterial activity was found. With the increasing of La content increased to 0.11 wt%, 0.19 wt% and 0.42 wt%, the antibacterial rate increased to 54–62%, 78–89% and >99.6% against E. coli and S. aureus, respectively. However, all these alloys exhibited antibacterial function only to the sessile bacteria rather than surrounding planktonic bacteria.
The processing process of antibacterial stainless steel also greatly influences the finial antibacterial properties. 304 stainless steel prepared by strip casting and cold rolling only caused 65% of E. coli bacteria death upon direct contact for 24 h even if the copper content was as high as 18 wt%, much less than the recommended value of 90% [205]. As-received Cu-containing stainless steels did not have antibacterial ability and solid solution treatment could not provide with strong antibacterial ability [213]. Only the sample underwent solid solution and subsequent ageing treatment to possess precipitated phase, showed a distinct antibacterial function [200,218]. It has been widely accepted that the antibacterial function of Cu-containing stainless steels was attributed mainly to the ε-Cu precipitate during heat treatment. Solid solution dissolves Cu element in matrix completely, thus the bacteria will not contact with Cu-rich compound, which will reduce the antibacterial activity significantly. After ageing treatment, nano-scale Cu-rich precipitates from the matrix and will be contact with bacteria during incubation, and kill the bacteria by some ways [220].
Further research displayed that aging temperature and duration had influence on the antibacterial properties. The aging temperature should be high and the aging duration should be long enough for the complete precipitation of nano-scale Cu-rich phase from the matrix and thus resulting in a high antibacterial activity. In Wang's study [219], after being aged at 500 °C for 4 h, 420-3.5Cu alloy only had an antibacterial rate of 69.2% against S. aureus, while the ageing treatment at 500–800 °C for 6 h improved the antibacterial rate significantly to as high as > 94%. Similar results were also reported elsewhere, when the aging duration was extended to 240 min at 700 or 800 °C, even the addition of as low as 1.5 wt% Cu generated enough Cu-containing precipitates by long-time diffusion, to make the antibacterial rate reach at > 99.99% against S. aureus [203].
It was thought that solid solution treatment could provide strong antibacterial ability for Ag-containing stainless steels [209,223]. Ag and Fe have a low mutual solubility in both the solid and the liquid states, and the silver solidified and reacted with impurities forming a silver-rich compound during solidification to obtain strong antibacterial ability [223]. Compared to the conventional Cu- or Ag- bearing stainless steels, the Ce-bearing stainless steels exhibited good antibacterial ability after solid solution treatment, which was attributed to the precipitation of Ce-rich zones in the solid solution treated sample due to the low solubility of Ce [108].
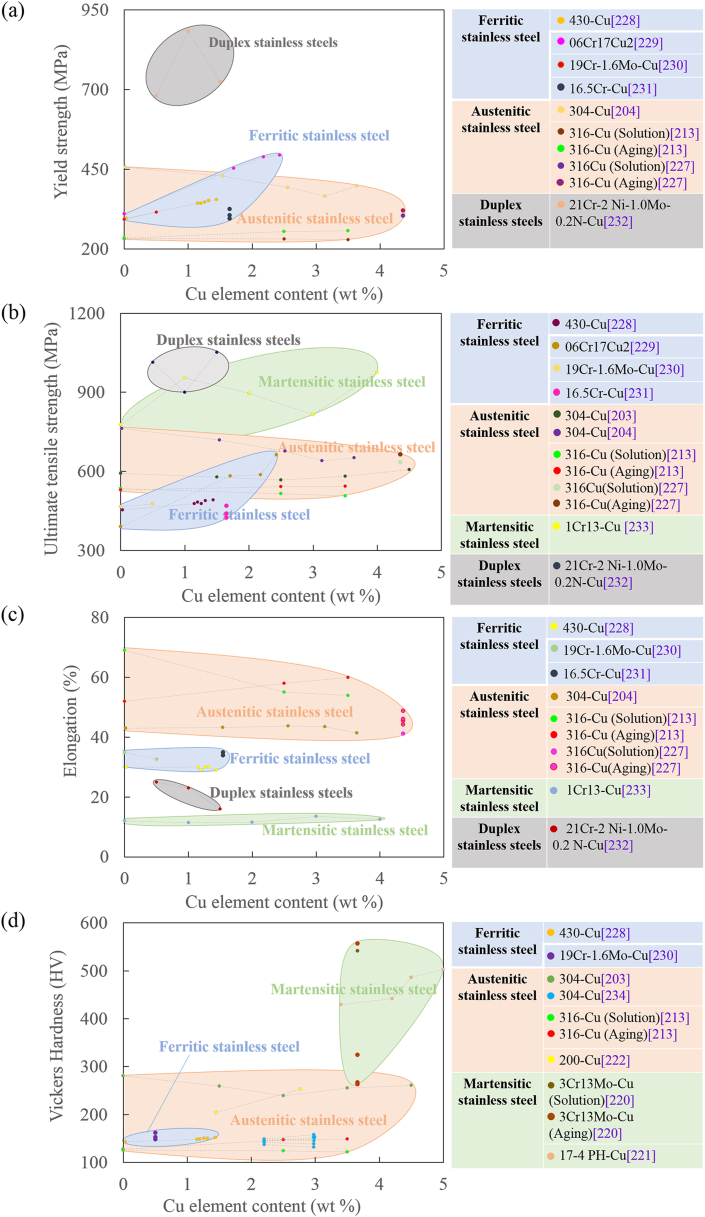
6.2. Mechanical properties
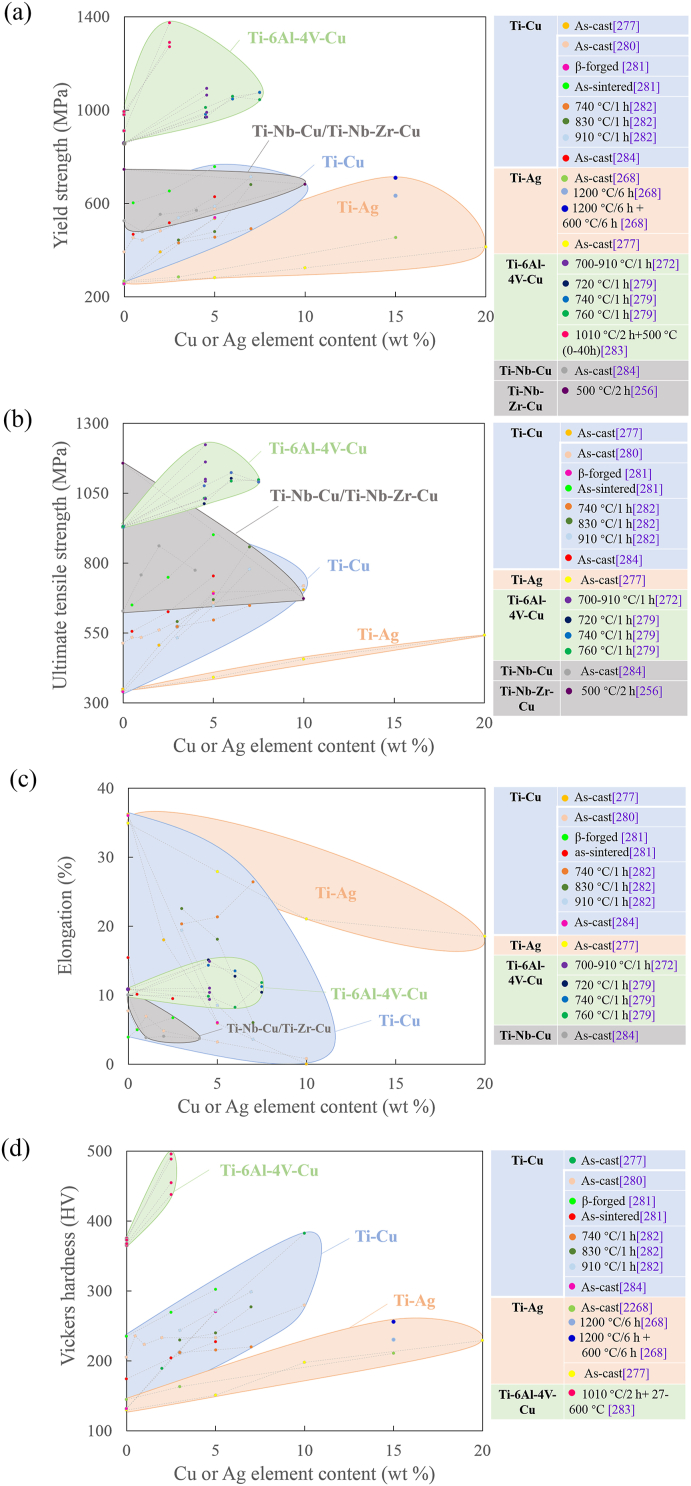
Cu and Ag elements have very low solid solubility in Fe matrix at room temperature. Cu and Ag normally do not form intermetallic phase with Fe matrix and exist in Fe matrix as solid solution state or Cu- or Ag-rich phase. It was reported addition of sufficient Cu could increase mechanical properties (including strength and hardness) such as adding over 2.5 wt% Cu into 304 [203]. Xi [213] reported the strengthening effect of Cu was limited when the Cu content reached at 2.5% for 316L-Cu no matter solution or aging treated, indicating Cu addition with 2.5 wt% was enough to precipitate sufficient Cu-rich phase for precipitation hardening. It was reported that low Cu addition (1.45 wt %) did not bring influence on hardness during 700 °C aging treatment, but high Cu content (2.77 wt%) significantly increased the hardness due to the precipitation of Cu rich phase [222]. In another research, the Vickers hardness of 17-4 PH-Cu stainless steel was positively related to the Cu content (3.4–5.06 wt%) [221]. Fig. 10 summaries the relationship between mechanical properties of antibacterial stainless steels and the Cu or Ag content.
Fig. 10.
Relationship between Cu content and mechanical properties of stainless steel (data from Refs. [203,204,213,[220], [221], [222],[227], [228], [229], [230], [231], [232], [233], [234]]). a) yield strength b) tensile strength, c) elongation d) hardness.
It was also suggested that the addition of Cu had tiny influence on the mechanical properties of solution treated stainless steel, which were mainly attributed to the synergistic effect of the softening effect by increase of stacking fault energy (SFE) and strengthening effect by the solid solution strengthening [213]. Nano-scale Cu-rich precipitation caused by the saturated Cu in the steel in the aging treatment played a positive role in mechanical properties [213,222]. The yield strength of 316L-Cu was almost similar to that of 316L after solution treatment, whereas the aging treatment obviously increased the yield strength of 316L-Cu due to the formation of tiny Cu-rich precipitates [213,231].
Extension of the aging time after solution treatment can enhance the mechanical properties of antibacterial Cu-bearing stainless steels without impairing antibacterial properties, while hardly changes mechanical properties under exceeding critical ageing time [219,220]. As the aging time extended, the hardness of 200–2.77Cu increased rapidly and approached a peak value after 3 h, and then hardly changed [222]. In Xi's study [231], the mechanical property of 316L-4.36Cu remained steady with the aging time increasing from 20 min, further even up to 15 h, implying the modest precipitation hardening effect of Cu-rich precipitates.
It was reported the aging temperature could also affect the mechanical properties of antibacterial Cu-bearing stainless steel. As the aging temperature increased within 500–800 °C, the softening effect and the dislocation density decreased rapidly, resulting in a rapid decreased in the hardness of the 420-Cu steel [219]. Similarly, when the aging temperature increased from 500 °C to 800 °C, the strengthening and hardness of 3Cr13Mo–Cu gradually decreased, which was attributed to the Cu segregation zone in steel evolved into precipitated particles, and the pinning effect of Cu-rich phase on dislocation is weakened [220].
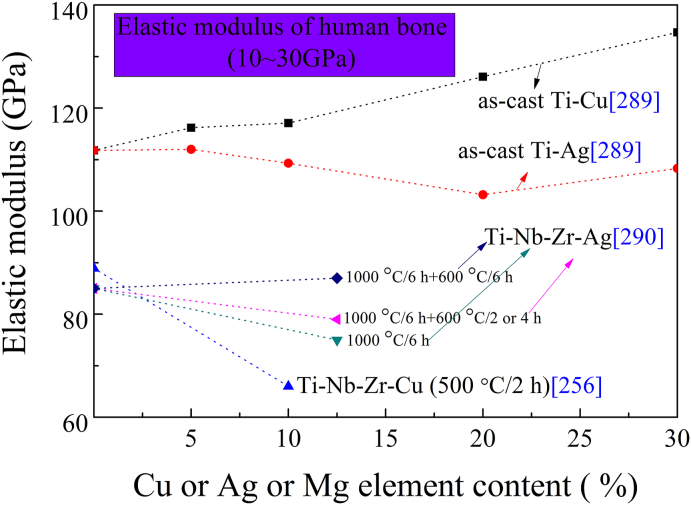
Wang [211] fabricated a novel type of low stiffness porous 316L-4.5Cu by selective laser with similar stiffness (3–20 GPa) with bone to prevent stress shielding. Such low stiffness porous structures, especially coupled with the addition of antimicrobial Cu, may provide a new direction for medical stainless steels.
6.3. Corrosion properties
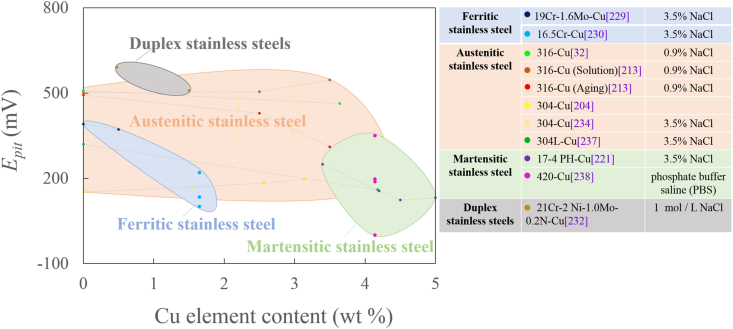
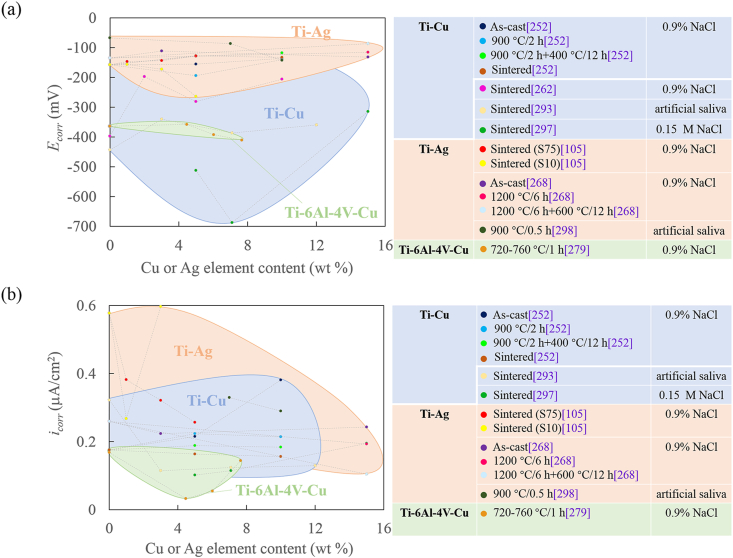
Long-lasting and broad-spectrum antibacterial activity of antibacterial stainless steel is inseparable from corrosion resistance. It is widely accepted the metal ion release, including Cu and Ag ion, controls mainly the antibacterial activity [206,235]. Thus, it is reasonable to believe that high metal ion release will lead to high antibacterial ability. The high metal ion release concentration on the other hand might cause cell toxicity. Therefore, one main challenge in the designing and manufacturing biomedical antibacterial stainless steels is how to optimize the chemical composition, especially the Cu content or Ag content, to balance the corrosion resistance, antibacterial property and cell compatibility. The research on the resistance of Cu-containing ASS to localized corrosion in humans (simulated with 0.9% NaCl solution or PBS buffer) is multiple, but has not been clarified sufficiently, and there are some controversial conclusions in different literatures [236]. Fig. 11 summaries the pitting corrosion potential (Epit) of antibacterial stainless steels.
Fig. 11.
Relationship between the Cu content and the pitting corrosion potential (data from Refs. [32,204,213,221,229,230,232,234,237,238]).
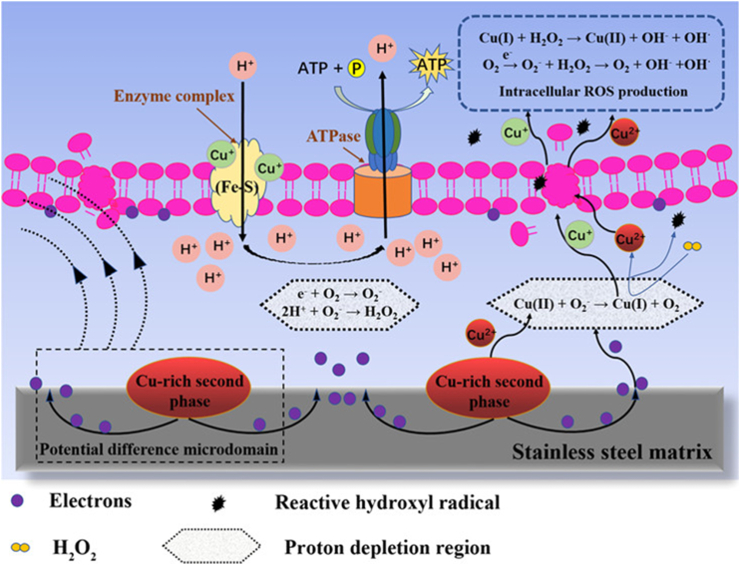
The corrosion properties of Cu-containing antibacterial stainless steels are closely related to the heat treatment process. It was reported that the Cu addition enhanced the corrosion resistance in the solution state under the mild corrosion environment (simulated with 0.9% NaCl solution or PBS buffer, 37 °C), whereas the corrosion resistance was detrimental after aging treatment [213]. After solution treatment, dissolved Cu ions in the surface would form a protective CuCl2 film in the chloride media, and was beneficial to improvement in the corrosion resistance of 316L-Cu [213]. However, in order to obtain high antibacterial ability, the antibacterial stainless steel has to be aged to precipitate Cu-rich phase from the matrix. Many researchers insisted the Cu-rich phase precipitated from a saturated amount of Cu in the steel matrix after the aging treatment formed galvanic corrosion with steel matrix and acted as a cathodic center, thereby accelerating the dissolution of the matrix [32,206,218]. The overall reaction was [32,221,239].
| (3) |
Cu-rich precipitates would destroy the uniform continuity and compactness of the passive film and act as the “weak points” on the steel surface which are susceptible to the local attack. As a result, the discontinuity of passive film caused by Cu-rich precipitates reduced the resistance to pitting corrosion and the ability of self-passivation and self-repairing [218,240]. Sun [32] reported that the addition of copper after aging treatment slightly decreased the pitting potential (Epit) of 317L, indicating the copper addition influenced the structure of the passivation film.
The aging time also has a certain effect on the corrosion resistance of the antibacterial stainless steel. Ren [241] reported that the radius of the capacitance arc in the Nyquist diagram of 304-3.9Cu was reduced gradually with the increase of the aging time, which indicates that formation of the passive film on surface of the steel was affected by the Cu precipitate in the steel, and thus the corrosion resistance of the steel was reduced to some extent. In another research, 304L-Cu possessed a higher intergranular corrosion tendency and a higher pitting corrosion rate with extended aging time [239].
The Cu content also has a great influence on corrosion performance of antibacterial stainless steels. It was reported that the increase of copper content in Fe–18Cr–Cu could increase the tendency toward chromium segregation at grain boundaries and thus the tendency toward intergranular corrosion of cast alloys [240]. In Xi's study [213], the corrosion resistance increased as the Cu content (0, 2.5%, 3.5%) increased for the solution-treated 316L, while opposed for the aged steel. In those higher Cu-content alloys that contain Cu-rich precipitates, the anticorrosion performance of the steel was greatly deteriorated, with the corrosion mechanism changing from pitting corrosion to selective corrosion [205]. Wang [221] reported the potentiodynamic polarization curve of Cr17Ni4–Cu in 3.5% NaCl solution shifted to the more negative direction, and Epit and ipit were more negative with increasing Cu content, which was believed to be related to Cu-rich phase formed after ageing treatment.
However, few researches indicated that the Cu had no adverse effects on the corrosion resistance of antibacterial stainless steels under the mild corrosion environment of 0.9% NaCl solution. The effect of trace Cu is similar to that of nickel, which will improve the corrosion resistance of weathering steel by forming a dense protective rust layer on the surface [119]. Nan [222] reported the passivity domains were very close between 200 and 1.45Cu with low copper content and 200–2.77Cu with high copper content after aging treatment at 700 °C, indicating that addition of Cu in the 200 stainless steel did not have strong influence on the corrosion resistance.
Antibacterial stainless steels showed excellent corrosion behaviors in bacterial solution. The addition of Cu or Ag improves the pitting corrosion resistance of stainless steel [210,242,243]. It was suggested that Cu-rich precipitates will be changed to Cu2+ partially due to the interaction between the materials and bacterium in the bacterial solution, which should reduce the conductivity of the surface of the steel, and result in a lower icorr of antibacterial stainless steels [207,243]. The presence of Cu in the 304L-Cu SS inhibited the pitting corrosion due to E. coli [242]. The pitting corrosion resistance of 2205-Cu was significantly better than that of 2205 in the presence of P. aeruginosa [243]. It was reported that Ag containing 304 (0.039Ag) restricted bacterial (P. sp) adhesion compared to the normal stainless steel, which reduced the pitting corrosion [210]. Yuan [244] reported the microbiological (S. aureus) corrosion resistance of 316L containing an appropriate amount silver was improved, but an excessive silver was prone to form segregation and degrade the corrosion resistance. In addition, La and Ce were believed to improve the anticorrosion performance of 316L in a medium containing sulfate reducing bacteria (SRB) [148].
6.4. Biocompatibility
The metal ion release from antibacterial stainless steels plays a very important role in the antibacterial behavior. So far, the research results have demonstrated that Ag- or Cu-containing stainless steels have good biocompatibility in vitro similarly with traditional biomedical stainless steels, which offers a prerequisite for the in vivo (animal test) and provides an important judgement for the development and application of antibacterial stainless steels.
The results of cell compatibility of antibacterial stainless steel in vitro are summarized in Table 6, results clearly showed the cells viability of Ag- or Cu-containing antibacterial stainless steels was improved or not significantly different compared to stainless steels, indicating that Ag- or Cu-containing antibacterial stainless steels had no cytotoxicity to MG-63 cells [16,214], KB cells [16], rMSCs [211], UECs [215,245], SaOS-2 cells [246] and MC3T3-E1 cells [216]. Moreover, the apoptosis rates to MG-63 [16], KB cells [16], UECs [245], SaOS-2 [246] of Ag- or Cu-containing antibacterial stainless steel decreased or had no significant difference compared with the control stainless steel. It can be found 316L-Cu SS behaved the fast in cells migration, which was beneficial to the endothelialization [215]. In addition, Ren [246] reported 317L-Cu alloy could promote the osteogenic differentiation by stimulating the alkaline phosphatase enzyme activity and the osteogenic gene expressions (including Col1a1, OPN, and Runx2).
Table 6.
The cell biocompatibility of antibacterial stainless steel.
| Test | Alloy system | Year | Cell line | Method | Result (antibacterial stainless steel compared with stainless steel) | [Ref.] |
|---|---|---|---|---|---|---|
| Cell viability | 317L-4.5Cu | 2011 | MG-63 | MTT | significant cellular growth | [16,214] |
| 2015 | SaOS-2 | MTT | no significant difference | [246] | ||
| 2016 | MC3T3-E1 | real-time cell analysis (RTCA) | no significant difference | [216] | ||
| 304-3.9Cu | 2013 | MG-63; KB | CCK-8 | no significant difference | [16] | |
| 316-4.5Cu | 2016 | rMSC | MTT | no significant difference | [211] | |
| 2016 | UEC | MTT | no significant difference | [215,245] | ||
| 2018 | UEC | MTS | significant cellular growth | [215,245] | ||
| Cell apoptosis | 304-3.9Cu | 2013 | MG-63; KB | Annexin V-FITC/PI staining | no significant difference | [16] |
| 317L-4.5Cu | 2015 | SaOS-2 | Annexin V-FITC/PI staining | significantly lower | [246] | |
| 316-4.5Cu | 2018 | UEC | Annexin V-FITC/PI staining | significantly lower | [215,245] | |
| Cell differentiation | 317L-4.5Cu | 2015 | SaOS-2 | ALP | mildly higher | [246] |
| cells migration | 316-4.5Cu | 2016 | UEC | wound healing assay | more cells migrated toward the wound | [215,245] |
Compared with the conventional 316L, Cu-containing 316L was proved to inhibit the inflammation reaction caused by endothelial dysfunction through blockading the inflammatory factors (TNF-α, IL-1β, 6, 8) to reduce the in-stent restenosis after the stent implantation, which would reduce the recruitment and infiltration of leukocytes, rather than have direct effect on leukocytes [247]. In addition, anti-fibrotic function of 316L-4.5Cu could reduce recurrence of ureteral stricture after stent implantation [248].
The continuous release of Cu2+ ions from 316L-Cu stainless steel will contribute to the Cu/Zn superoxide dismutase viability, which steadily reduces toxicity and encrustation [245]. Some researchers [215,249] reported 316L-4.5Cu had satisfied dual functions with anti-encrustation and anti-infection.
6.5. In vivo test
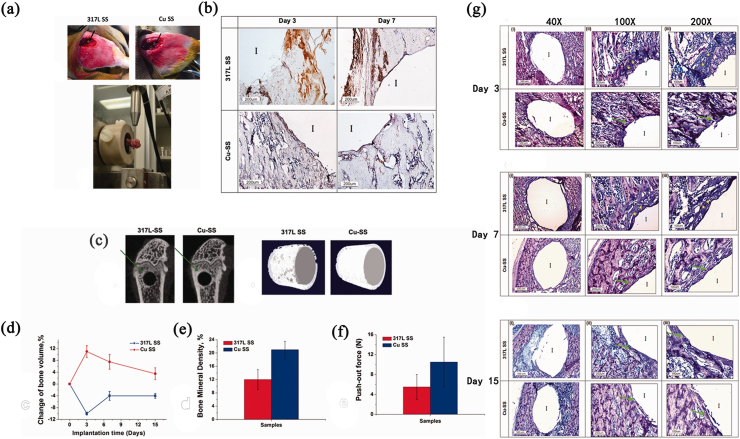
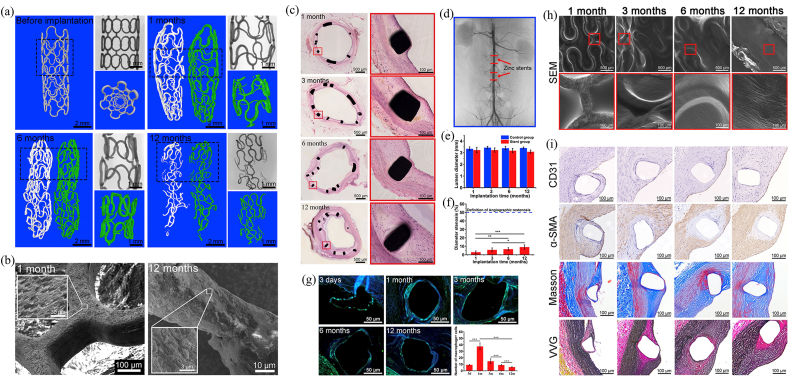
Antibacterial stainless steel has not been applied clinically, but 317L-4.5Cu and 316L-3.77Cu have been tested in vivo. The in vivo test results have demonstrated the good biocompatibility of antibacterial stainless steel, as listed in Table 7.
Table 7.
In vivo results of antibacterial stainless steel.
| Year | Antibacterial stainless steel | Animal model | In vivo results | [Ref.] |
|---|---|---|---|---|
| 2011 | 317L-4.5Cu | Japanese white rabbits | Strong antimicrobial activity, anti-inflammatory ability and strong promoting osteogenesis in vivo:
|
[214] |
| 2014 | 317L-4.5Cu | Sprague-Dawley rats | Strong anti-inflammatory ability, superior bone-implant integration and promoting osteogenesis in vivo:
|
[246] |
| 2016 | 317L-4.5Cu | zebrafish embryos | No in vivo cytotoxicity:
|
[32] |
| 2017 | 317L-4.5Cu | Sprague-Dawley rats | Promoting osteogenesis:
|
[250] |
| 2020 | 316L-3.77Cu | Sprague-Dawley rats | Implant-related infection was also alleviated by 316L-Cu SS | [212] |
It was suggested from current in vivo results that Cu-containing ASS showed strong anti-infection and anti-inflammatory activity [212,214,246] as well as the ability of promoting osteogenesis [214,246,250] in vivo, as Fig. 12. Moreover, the push-out of Cu-containing ASS implant was doubled compared with that of 317L SS (p < 0.05), suggesting Cu-containing ASS implant demonstrated superior bone-implant integration in animal model [246], as Fig. 12(f). Cu-containing stainless steel was also reported to promote fracture healing by accelerating the callus evolution process. However, the specific mechanism needed to be further explored [250]. Ren [246] further proposed Cu2+ ions released from the Cu-containing stainless steel did have promotion effect on osteogenesis through three successive courses including osteoinduction, osteoconduction, and osseointegration, which are the most important factors to the osteogenic behavior for implants.
Fig. 12.
The in vivo results of copper-containing antibacterial stainless steel [246], copyright 2015, John Wiley and Sons, Inc. (Wiley Company). (a) Surgical procedures and set-up of the biomechanical push-out test. Black arrows show the implant positions (b) Immunohistochemical staining of TNF-α of bone tissue around the implants. Brown color represents positive staining and “I” the implant location. (c) Micro-CT images and Micro-CT 3D reconstruction models after 15 days of postoperation. (d, e) Bone volume, bone mineral density of the bones formed around 317L SS and Cu-SS implants. (f) Average maximum push-out forces of 317L SS and Cu-SS implants after 15 days of implantation obtained by biomechanical push-out test. (g) Histological photographs of Giemsa stained bone tissue formed around the implants.
The excellent and unique antibacterial stainless steel is looking forward to being widely used in the biomedical application, such as orthopedics, dental and other hard tissue repair and replacement, cardiovascular stent intervention, ureteral stents, and manufacturing medical devices to ensure safety and hygiene. Another possible application of antibacterial stainless steel is surgical instruments, orthopedic furniture and outside surface of clinical equipment because of its excellent antibacterial activity against the biofilm, good biocompatibility with animals, and strong ability of corrosion resistance.
7. Antibacterial titanium alloys
Titanium and titanium alloy have been widely used in long-term implants, such as spine screw and spine fusion cage due to their good biocompatibility. In the long-term implantation, infection or inflammation will be a serious problem due to the good surface biocompatibility of titanium and titanium alloys, therefore, titanium alloy with antibacterial properties should have great potential. Here are some important turning points for the development of antibacterial titanium alloys, as illustrated in Fig. 13.
Fig. 13.
Development flow chart of antibacterial titanium alloys [17,18,71,74,104,105,195,[251], [252], [253], [254], [255], [256], [257], [258]].
The first report on antibacterial titanium alloy was published in 2009 [17]. The results demonstrated that Ti–1Cu possessed excellent osteoid-formation and no cell toxicity and thereby was promising to be a biomaterial [17]. The first report on the antibacterial properties of Ag-containing titanium alloys was published in 2012 [104]. Antibacterial Cu-containing and Ag-containing titanium alloys are presently two main alloy systems of antibacterial titanium alloys. During the past ten years, many great achievements have been obtained on the development of new alloy systems, the optimization of antibacterial activity and mechanical properties, cell biocompatibility in vitro and in vivo as well as antibacterial mechanism. Recently, antibacterial titanium alloy with low elastic modulus was reported, which exhibited strong antibacterial ability (>90% reduction) and low elastic modulus of 66 GPa, close to β- or near β-titanium alloy [256]. This alloy shows the potential ability to reduce the infection and “stress shield” for bone implant application.
Although the addition of copper or silver element provides with strong antibacterial activity against bacteria, the antibacterial titanium alloys are still inert to cells. Surface biomodification is still need to improve the surface cell adherence and proliferation even osseointegration. Surface acid etching (SAE) [74], sand blasting, sandblasting and acid etch (SLA) [75,76,104], anodization [259,260], micro-arc oxidation (MAO) [73] etc. have applied to antibacterial titanium alloy. So far, only several in vivo research reports have been published concerning the bone implant and dental implant. More in vivo researches should be carried out to provide more clinical evidences for the advantage of antibacterial titanium alloy instead of pure titanium and Ti–6Al–4V and promote the clinical application of antibacterial titanium alloy. Fig. 14 shows the research interests in the antibacterial titanium alloys.
Fig. 14.
Research activities in antibacterial titanium alloys.
7.1. Antibacterial properties
The research results of Cu-containing and Ag-containing antibacterial titanium alloys in recent years are listed in Table 8, suggesting the antibacterial titanium alloys exhibit excellent broad-spectrum antibacterial properties against lots of bacteria, such as S. aureus, E. coli, P. gingivalis, S. epidermidis, S. mutans as well as S. sobrinus. Antibacterial titanium alloy has different resistance to different bacteria. For example, S. aureus displayed markedly less adhesion on Ti–Cu antibacterial alloy compared with E. coli [17,254]. It was also noticed Ti–Cu sintered alloy showed certain anti-anaerobic bacteria activity, which is much less than their anti-aerobic bacteria abilities [194]. The antibacterial titanium alloy currently developed is mainly targeted at E. coli and S. aureus which are most common causes of implant-associated infections.
Table 8.
Cu-containing antibacterial titanium alloys.
| Alloy system | Bacteria (antibacterial rate[Ref.]) |
|---|---|
| Ti-xCu (x = 1–25 wt%, 57.03 wt%) |
S. aureus (33%–100% [18,252,255,[261], [262], [263], [264], [265]], 100% after incubation for 12 h [258], No [17,193,195,262]); E. coli (49.3%–99.9% [18,255,[261], [262], [263],265,266], 100% after incubation for 12 h [258], No [17,262]); P. gingivalis (54.4%–75.4% [194]); S. epidermidis (4%–27% at 6 h [267]); S. mutans, P. gingivalis [52] |
| Ti-xAg (x = 1–30 wt%) |
S. aureus (3%–99.9% [71,74,105,268], 91%–98% after 30 days [259]); S. mutans, S. sobrinus [269]; |
| Ti–6Al–4V-xCu (x = 1–6 wt%) |
S. aureus (51%–99.46% [6,253,254,[270], [271], [272]], No [273]); E. coli (22%–99% [253,254,274], No [273]); |
| Ti–6Al–4V-0.5Ag | E. coli (75% [274]); |
| Ti–13Nb–13Zr–10Cu | S. aureus [256] |
| Ti–Nb-xAg (x = 0.2–1.2 wt%) | S. aureus (92.66%–100% [275]); E. coli (71.4%–100% [275,276]) |
Note: 24 h incubation as default.
“No” means no antibacterial rate is shown in the literature.
It is now widely accepted that Ag- or Cu-containing particles in Ti–Ag or Ti–Cu alloy control mainly the finial antibacterial activity while Ag ion or Cu ion release also contributes the improvement in the antibacterial properties. According to this conclusion, any measure which could promote the formation or the precipitation of Cu- or Ag-containing compounds will enhance the antibacterial activity. Cu or Ag content influences the antibacterial property and high content normally results in a high antibacterial activity [105,194,262,267,273]. However, it is noted that no antibacterial property was not reported on the as-cast Ti–Cu alloys with up to 10 wt% Cu [252] or as-cast Ti–Ag alloys with up to 30 wt% Ag [268,269].
Although Ti–Ag and Ti–Cu are similar alloy system, but the solid solubilities of Cu and Ag element in α-Ti are quite different. For example, the maximum solid solubility in α-Ti is about 2.1 wt% of Cu at 790 °C and about 10 wt% of Ag at 855 °C, respectively. Recently study on Ti–Ag alloy demonstrated that nano-scale and homogeneously distributed Ti2Ag phases plays the key role in the antibacterial activity of Ti–Ag alloy [71]. Therefore, more Ag is need for Ti–Ag alloy to obtain Ti2Ag phase by ingot metallurgy and heat treatment. Some studies also demonstrated that certain surface treatments would further improve antibacterial properties of Ti–Ag alloy [74,259], in which one of the most representative technologies is acid etching, and the particles with high Ag contents are thought to play a key role in the antibacterial mechanism [74].
Heat treatment has great influence on the antibacterial properties of Ti–Ag and Ti–Cu. Recently, more research results confirmed the influence of Ti2Cu phase or Ti2Ag phase on the antibacterial activity of Ti–Cu and Ti–Ag alloy, and found that high volume fraction of Ti2Cu phase (or Cu-rich phase) and Ti2Ag phase (or Ag-rich phase) normally results in a high antibacterial rate [71,105,252,264,266,268]. Heat treatment process can mainly change the existing form of Cu or Ag element, in turn affect the final antibacterial activity of titanium alloy. Cu or Ag element almost completely dissolved in matrix in an interstitial atoms condition after solution treatment followed by a fast cooling rate such as water cooling, thus the treatment had little improvement on antibacterial property of Ti–Cu or Ti–Ag alloy [252,268]. However, it was reported after solution treatment followed by a slow cooling rate such as air cooling or furnace cooling, the Ti–Cu alloy had antibacterial property against E. coli, S. aureus, S. mutans as well as P. gingivalis, which was because Ti2Cu precipitated from titanium matrix when the cooling rate was slow after solution treatment as Cu was a fast eutectoid element in Ti–Cu alloy [52,255,263]. The aging treatment significantly improved the antibacterial property due to large amount of nano-scale Ti2Cu or Ti2Ag precipitated from matrix [252,268]. Fortunately, it has been recognized Ti–Cu alloys and Ti–Ag alloys with sufficient Cu or Ag content had antibacterial property after heat treatment. The addition of 15 wt% Ag was enough for Ti–Ag alloy after solution treatment and aging treatment to have a much high antibacterial rate (>99.9% reduction against S. aureus) [268]. However, it is controversial whether Ti–3Cu alloy has antibacterial property in aging state. Hu [73] indicated Ti–3Cu alloy in the aging state showed no antibacterial activity against S. aureus with 60–80% antibacterial rate. But in previous researches, Ti–3Cu alloy in the aging state had antibacterial property against S. aureus (>90%) [193,264]. It has been now recognized that addition of 5 wt% Cu was enough for Ti–Cu alloy after proper heat treatment had excellent antibacterial property [52,252,255,263].
In powder metallurgy, Ag element mainly reacts with Ti to form Ti–Ag intermetallic compound and very little dissolve in Ti as interstitial atoms. Thus, it is possible to prepare antibacterial Ti–Ag alloy with low Ag content by powder metallurgy. It was only required to contain 3 wt% Ag for Ti–Ag sintered alloys (10 μm Ag powder) in order to obtain a strong and stable antibacterial property against S. aureus (98.2%), which was attributed the precipitation of a large amount of micro-scale Ti2Ag [105]. In addition, Chen [105] demonstrated the reduction in the Ag powder size contributed to the improvement in the antibacterial property, which was attributed mainly to the smaller particle size silver powder is easier to form fine and homogeneous Ti2Ag during sintering. Similarly, Cu content had to be ≥ 5 wt% for Ti–Cu sintered alloys in order to obtain strong and stable antibacterial property against S. aureus and E. coli due to the precipitation of a large amount of micro-scale Ti2Cu phase [252,262].
The antibacterial property of as-cast Ti6Al4V Cu alloy has not been reported, and the heat treatment has an effect on the antibacterial property. The results indicated that appropriate solution treatment could make Ti6Al4V–Cu alloy have antibacterial properties [270,272]. Ma [270] reported Ti6Al4V–5Cu alloy solution treated at 810–1050 °C for 1 h and followed by water quenching had antibacterial activity against S. aureus (90.2%–98.6%), and the higher the solution temperature was and the stronger the antibacterial ability was. This probably because sterilized Cu ions was easier released from interstitial atoms compared with the large number of stable Ti2Cu phase precipitated during the aging process. Peng [272] investigated the Ti6Al4V5Cu treated at low solution temperature of 700 °C and 740 °C and followed by air quenching possessed good antibacterial property (95.4–97.5%), while treated at high solution temperature of 780–910 °C and followed by air quenching did not possess antibacterial ability (only 51.0%–77.2%). The authors speculated the larger globule Ti2Cu or bulk β phase in the alloy solution treated at low temperature, rather than the lathy Ti2Cu/α lamellas in the alloy solution treated at high temperature, could efficiently inhibit the growth of bacteria that adhered on the surface of the alloy. In other researches [271,273], it was also reported Ti6Al4V–5Cu alloy displayed strong antibacterial ability (>99% against S. aureus and E. coli) after solid solution and subsequent aging treatment. Therefore, it was only required to contain 5 wt% Cu for Ti6Al4V–5Cu alloys after proper heat treatment to obtain a strong and stable antibacterial property.
Moreover, A series of antibacterial Ti6Al4V–Cu were successfully fabricated by selective laser melting (SLM) technology [253,254], which shows the possibility of Cu -containing antibacterial titanium by additive manufacturing. Research of Ti–6Al–4V-xCu prepared by SLM confirmed that the increase in Cu content enhanced the antibacterial activity and Cu content had to be 4 wt% or more in order to obtain high antibacterial activity against E. coli and S. aureus (>90%) [253]. However, in another research, Ti–6Al–4V–5Cu prepared by SLM had no antibacterial property (22% against E. coli), which might be due to the fact that only a small amount of Ti2Cu was contained in the SLM materials manufactured in the study [274]. In addition, it appears that the addition of 0.5 wt% Ag is insufficient to provide antibacterial property (75% against E. coli) for SLM-titanium alloy [274].
Ag element was added to Ti–Nb alloy in order to obtain a titanium alloy with low modulus and antibacterial activity, and the antibacterial activity was attributed to nanoscale silver precipitates after solid solution and aging treatment [275,276]. It was reported that Ti–12Nb–1Ag alloy showed an antibacterial activity of 71.4% against E. coli [276]. But for Ti-27.5Nb alloy [275], the addition of 0.2–1.2 wt% Ag provided with very strong antibacterial activity (>90% against both S. aureus and E. coli). With the increasing of Ag content, the antibacterial activity increased and reached at 100% at 1.2 wt% Ag.
7.2. Mechanical properties
The strength of cp-Ti is insufficient for certain biomedical application, such as dental implants and dental prostheses [277]. Element alloying with silver or copper can enhance the mechanical strength, hardness, and wear resistance, however, it is always accompanied with a reduction in ductility [262,272,277]. Ti–Ag and Ti–Cu alloy are both eutectoid alloy system, which means that both Ag and Cu elements have solid solution strengthening ability and precipitation/dispersion strengthening ability [252]. Thus, the addition of Cu or Ag and the precipitation of Ti2Cu or Ti2Ag will improve the hardness and strength but might reduce the ductility of titanium alloy [277]. The entanglement dislocations in the alloy matrix further explain the strengthening of strength and wear resistance [278]. Thus, developing antibacterial titanium alloy with comparatively high strength as well as satisfied ductility to simultaneously meet strength need and avoid brittle fracture in clinical application is always desired. Fig. 15 summaries the relationship between mechanical properties of antibacterial titanium alloys and the Cu or Ag content.
Fig. 15.
Mechanical properties of antibacterial titanium alloys (data from Refs. [256,264,268,272,277,[279], [280], [281], [282], [283], [284]]). a) yield strength, b) ultimate tensile strength, c) elongation, d) hardness.
Alloying Cu or Ag element normally reinforce the as-cast cp-Ti matrix and enhance the hardness and the strength. The increasing in Cu or Ag content results in the improvement of strength and hardness of as-cast Ti–Cu alloy or as-cast Ti–Ag alloy due to the increasing in the volume fraction of Ti2Cu or Ti2Ag intermetallic particles while the ductility is opposite [252,277,285]. The hardness of as-cast Ti–Cu alloys are positively correlated with copper content (<10%) [252,277]. The tensile strength and hardness of as-cast Ti–Cu alloy increased with the increase of copper concentration. As the addition of copper increased from 5% to 10%, the tensile strength was basically unchanged, while the elongation declined from >6% to less than 1% [277]. It was suggested the addition of ≤20 wt% Ag improved the strength and hardness of as-cast titanium alloys while retaining relatively high elongation (≥19%) [277].
The heat treatment can improve the mechanical properties of Ti–Cu alloy and Ti–Ag alloy by changing the existence form and distribution of alloying elements Ag or Cu. Both solution treatment and aging treatment enhance the strength and the hardness but deteriorate the ductility of Ti–Cu alloy and Ti–Ag alloy, displaying the strong solid solution strengthening ability of Cu (or Ag) element and the dispersion strengthening ability of nanoscale Ti2 Cu (or Ti2Ag) [252,268,285]. Moreover, the strengthening increases with the increase of Cu (or Ag) content, which was attributed to the high Cu (or Ag) content resulted in a high volume fraction of Ti2Cu (or Ti2Ag) and high solid solution of Cu (or Ag) [252,268]. The proper heat treatment is very important for the mechanical properties of Ti Cu and Ti Ag alloys. For example, the strength and hardness of Ti–20Ag alloy solid solution treated at 1100 °C was greatly improved compared with that treated at 950 °C, even higher than 800Mpa, while the ductility was greatly reduced to less than 2%, indicating the alloy was not suitable for solution treatment at high temperature [285]. The tensile strength of Ti–3Cu alloy increased as the aging time increased from 16 h to 24 h, however, while hardly further increased when the aging time extended to 36 h, but the elongation had a greater decrease of 6.07%, which indicated too long aging time might make the grain coarsening and then reduced the properties [264].
Addition of 1% Cu into as-cast Ti–6Al–4V did not change the tensile strength and the elongation but further increasing of Cu decreased significantly the tensile strength and the elongation. Both elongation and tensile strength of the as-cast Ti–6Al–4V–Cu had negative correlation with copper content [286]. However, the mechanical properties of solution treated or aging treated Ti–6Al–4V–Cu were higher than that of similarly heat-treated Ti6Al4V alloy due to solid solution strengthening of Cu and the dispersion strengthening of Ti2Cu phase [272,283]. Hardness and tensile strength of Ti6Al4V–Cu alloy increased with increasing annealing temperature, but elongation was opposite [287]. Ti6Al4V–5Cu alloys showed good strength when treated at 700 °C–910 °C, while the elongation decreased from with the annealing temperature increased and only the alloys treated at 700 °C, 740 °C and 780 °C showed acceptable ductility [272]. Moreover, it was reported the hardness of Ti6Al4V-2.5Cu alloy increased with aging temperature up to 500 °C, indicating Ti2Cu was inadequate precipitation below 500 °C [283].
The metal preparation process and the metal forming process also have great influence on the mechanical properties. It is widely accepted Ti–Ag or Ti–Cu sintered alloy showed a much higher strength and hardness than cp-Ti but greatly reduction in the ductility [18,105,262]. In antibacterial Ti–Ag (or Ti–Cu) sintered alloys, nearly all Ag (or Cu)element reacted with titanium to form Ti2Ag (or Ti2Cu) phase during sintering process, and the high volume fraction of Ti2Ag (or Ti2Cu) phase contributed to the high hardness and strength [262,268]. Recently, Ti-57.03Cu was prepared by a powder metallurgy in which large amount of TiCu and Ti2Cu3 intermetallic phases with 23 nm grain size contributed to the ultra-high hardness of 10 GPa and acceptable toughness of 8.14 MPam1/2, which is much higher than other reported values in titanium alloys [265]. Further research demonstrated that hot forming processing, such as the extrusion, refined the microstructure and densified the alloy, in turn improved the ductility and strength without reduction in antibacterial properties [288].
More recently, additive manufacturing technology has been used to prepare metal alloy with high mechanical properties. It was reported antibacterial Ti–6Al–4V–Cu alloy fabricated by selective laser melting led to a substantial increase of the ultimate tensile strength compared with Ti–6Al–4V alloy but led to a decrease in ductility [254,274]. Research on Ti–Cu alloy prepared by additive manufacturing demonstrated much high tensile strength of 1073 MPa and acceptable elongation of 5.5%, owing to the formation of an ultrafine eutectoid microstructure [257], showing the great potential application in antibacterial titanium alloy and related implants.
There are few reports on antibacterial titanium alloy with low elastic modulus, as shown in Fig. 16. Cu and Ag elements were added to Ti–Nb or Ti–Nb–Zr alloy in order to obtain a titanium alloy with low elastic modulus and antibacterial activity. It was reported that the addition of Nb and Cu produced a higher strength than Ti–Cu and Ti–Nb binary alloys due to the solid solution strengthening of two elements and the precipitation strength by the dual-phase, but the elongation of the ternary alloys was only about 4%, much lower than the binary alloy [284]. Ti–13Nb–13Zr–10Cu alloy exhibited slightly lower compressive strength and yield strength than those of Ti–13Nb–13Zr alloy, but significantly higher than those of cp-Ti. Moreover, the alloy had an enhanced antibacterial activity against S. aureus as well as a low elastic modulus of 66 GPa, which might reduce the bacteria infection and “stress shield” in bone implant [256]. Cai [290] designed a novel Ti–13Nb–13Zr-12.5Ag alloy in aging state with low elastic modulus of 79 GPa and strong antibacterial ability (>90% against S. aureus). But for Ti-27.5Nb alloy, the addition of 0.2–1.2 wt% Ag provided with very strong antibacterial activity (>90% against both S. aureus and E. coli). With the increasing of Ag content, the antibacterial activity increased but the microhardness decreased [275]. Ti–12Nb–1Ag alloy showed an antibacterial activity of 71.4% against E. coli. However, no modulus or strength was available in this report [276].
Fig. 16.
Elastic modulus of Cu- or Ag-containing titanium alloys (data from Refs. [256,289,290]).
It was found that element alloying with silver or copper could improve the inherently poor grindability and wear resistance of titanium alloy due to the precipitation of eutectoid Ti2Cu or Ti2Ag phase [[291], [292], [293], [294], [295]]. Zhang [285] reported the wear resistance of Ti–Ag alloys increased with the increasing of Ag content (5–20% Ag). Kikuchi [291] indicated the grindability was significantly improved with an increase of the Cu content (0.5–10% Cu) in Ti–Cu alloy.
7.3. Corrosion properties
Good corrosion resistance is one of the most important reasons for titanium and titanium alloys to be applied as orthopedic and dental implants [296,297]. It is critical for antibacterial titanium alloys in biomedical application to judge whether the addition of antibacterial elements would affect the corrosion properties of titanium alloys in body environments. Fig. 17 summaries the corrosion properties of reported antibacterial titanium alloys. The corrosion behavior of titanium alloys is mainly controlled by a galvanic corrosion between titanium matrix and secondary phase in matrix. Both Cu and Ag element have higher standard electrode potential than titanium element, thus Cu–Ti and Ag–Ti intermetallic compounds normally have a higher electrode potential than titanium matrix. As a result, Cu–Ti and Ag–Ti intermetallic compounds normally act as cathode and titanium matrix acts as anode during the galvanic corrosion.
Fig. 17.
Electrochemical properties of antibacterial titanium alloys(data from Refs. [105,252,262,268,279,293,297,298]. a) Ecorr, b) icorr.
Addition of Cu into titanium alloys to manufacture as-cast Ti–Cu alloy containing small amount of Ti2Cu intermetallic compound would increase the strength and provide with strong antibacterial activity but do not facilitate the corrosion resistance due to galvanic corrosion [252,299]. The corrosion resistance of as-cast Ti–Cu alloy decreased with increasing of Cu content (0–15 wt% Cu), which could be attributed to more Ti2Cu phase leading to increasing of the galvanic corrosion effect [252,297]. However, it was reported that as-cast Ti–Ag alloys possessed higher corrosion resistance compared with cp-Ti [268,285,298]. In the as-cast Ti–Ag alloy, no or rare Ti2Ag precipitated from matrix due to the large solubility of Ag in α-Ti phase. Some researchers [298] attributed the enhancement in corrosion resistance to two reasons. Firstly, Ag is a nobler element than Ti thus can shift the corrosion potential of titanium alloys toward the noble direction. Secondly, corrosion will preferentially leach out Ti in preference to Ag, thus Ag atoms may accumulate on the surface of the Ti–Ag alloys and higher Ag content further increases the electrode potential of the alloys towards more positive values. But further increasing the Ag content to more than 20 wt%, the precipitated Ti2Ag and/or TiAg intermetallic compounds preferentially dissolved in 0.9% NaCl solution, which deteriorated the corrosion resistance [300].
The heat treatment including solution treatment and aging treatment can improve the corrosion resistance of Ti–Ag and Ti–Cu antibacterial titanium alloy, especially aging treatment. After solution treatment, Ti2Ag or Ti2Cu phases were dissolved completely in matrix, which could decrease the galvanic corrosion effect and increase the general corrosion resistance [252]. After aging treatment, lots of nano-scale second phases precipitates from matrix and produce “envelopes effect”, which envelopes the Ti-rich (less noble) phase to prevent the alloy from contacting corrosive liquid and thus provide corrosion protection [301]. It was reported that solid solution state Ag/Cu and massively nano-sized Ti2Ag/Ti2Cu particles in Ti matrix would improve the corrosion resistance [264,268]. In another hand, nanoparticles dispersed on surface could enhance corrosion resistance due to that their small size and high surface area lead to greater barrier properties, small nano-particles sizes tend to enhance anti-corrosion properties [302]. The addition of Cu in Ti6Al4V alloy would not affect its corrosion resistance. Solution treated and aging treated Ti–6Al–4V-xCu (x = 1, 3 and 5%) possessed better corrosion resistance than commercial medical grade Ti–6Al–4V alloy [273]. Ti6Al4V–5Cu solution treated at 740 °C and 910 °C showed relatively lower icorr and higher Ecorr compared with Ti6Al4V alloy [272].
Metal alloys preparation processing also has influence on the corrosion properties. Ti–Ag and Ti–Cu alloy prepared by powder metallurgy exhibited better corrosion resistance than cp-Ti, and the corrosion resistance increased with increasing the addition of Ag content (1–5% Ag) or Cu content (2–25% Cu) [105,262]. However, the size, number, and morphology of pores would affect the corrosion behavior of sintered alloy [303]. For example, inhomogeneous distribution of Ti2Ag phase in Ti–Ag sintered alloys prepared with Ag powder sized 10 μm compared with 75 μm resulted in more pores on the surfaces, accelerating corrosion rate [105]. However, in another research, the corrosion current density of Ti-57.03%Cu sintered alloy was about 3 times higher than that of cp-Ti [265]. Corrosion resistance of antibacterial Ti6Al4V-xCu (x = 0, 2, 4, 6 wt%) alloys fabricated by selective laser melting (SLM) technology were stronger than Cu-free alloy [253].
The metal ion release from antibacterial titanium alloy, such as Cu ion and Ag ion, affects the antibacterial activity but also influences the biocompatibility. In vitro results indicated that the release amount of Cu or Ag ions from antibacterial titanium alloy immersed in 0.9% NaCl solution were very low. It has been reported the Cu ion release of any antibacterial titanium alloys is much less than the recommended daily intake of Cu for an adult by WHO (2–3 mg). It was found that the existing forms of Cu element significantly affected the Cu ion release behavior in the following order from low to high: as-cast Ti–Cu < solution treated Ti–Cu < aging treated Ti–Cu < sintered Ti-Cu [252]. Ag or Cu ion release behavior is closely controlled by its alloying content and a high content normally corresponds to a high ion release [105,252,262]. It was also reported Ag ion concentrations from all Ti–Ag sintered alloys (1–3% Ag) after 24 h immersion were lower than 5 ppb, and Ag ion concentration release from Ti–5Ag sintered alloys was higher than that of Ti–1Ag and Ti–3Ag sintered alloys [105]. Zhang [252] reported high Cu concentrations from 7 ppb to 27.5 ppb were detected in Ti–5Cu alloys and Ti–10Cu alloys with different existing forms of Cu element in 24 h, and Cu ion concentration release from Ti–10Cu alloys was nearly 2 times higher than that of Ti–5Cu alloys. Moreover, the high release rate of Cu or Ag ions was only shown at the beginning of the immersion, which might be attributed to the formation of passive layer on the surface with the increasing immersion duration. Metal ion release rate decreases with the extension of the immersion duration. Zhang [18] reported Cu ion release concentration of sintered Ti–10Cu was 50 ppb after 72 h immersion. Liu [262] pointed out when the immersion time exceeded 72 h, the Cu ion release rate of the sintered Ti–Cu alloy decreased with the immersion time. Ma [304] reported Cu ion release rate of Ti–5Cu alloy in solution state was 8.3 ppb per day in the first 10 days but slowed down to 2.35 ppb per day from 10th to 30th day. Peng [272] pointed out that the total cumulative release of Cu ions from Ti6Al4V–5Cu alloys after 20 days immersion was only 3.108 ppb and 2.498 ppb, respectively. Ma [270] indicated Ti6Al4V–5Cu alloys after 90 days immersion released 244 ppb Cu ions.
In addition to 0.9% NaCl, the corrosion resistance of antibacterial titanium alloys in other solutions has also been widely studied. Varied fluoride-containing materials have been used in dental applications such as toothpaste and mouthwash to prevent dental caries [305,306]. However, fluoride ion is liable to react with titanium dioxide passivation film on the surface of titanium alloys, making the titanium potential more active and accelerating the corrosion rate [307,308]. Although artificial saliva solution containing fluoride ions significantly reduced the corrosion resistance of the Ti–Ag alloy, the corrosion resistance of the Ti–Ag alloy in as-cast and solid solution state was still higher than that of the pure titanium [285,298,309]. But it was reported Ti–Cu sintered alloy showed higher corrosion rate in artificial saliva containing F− compared with cp-Ti and increased with the increase of Cu content (3–10% Cu) [310]. Lactic acid is a metabolite produced when anaerobic bacteria in the oral decompose glucose, which can reduce the pH of body fluid and may be harmful to corrosion resistance of metal alloy. Unlike the as-cast Ti–Ag alloy in the NaCl solution that Ti2Ag was preferentially attacked, while in the lactic acid solution, Ti2Ag was not preferentially dissolved, thus insoluble Ti2Ag might reduce the speed of dissolution for the Ti–Ag alloys in the transpassive region [299,311]. Takahashi [299] reported that titanium with 20–25% Ag had excellent corrosion resistance in the lactic acid solution. After immersing in the lactic acid solution for 7 days, the amount of Ti ion release of the as-cast or the solution treated Ti–Ag alloy was significantly lower than that of cp-Ti, and the Ag ion was hardly released [285].
7.4. Biocompatibility
A certain amount of Ag ions or Cu ions may cause cytotoxicity, therefore, research on cell compatibility is essential for silver-containing or copper-containing antibacterial titanium alloys as biomaterials. At present, the biocompatibility of antibacterial titanium alloys in vitro is mainly focused on Ti–Cu alloy and Ti–6Al–4V–Cu alloy and few reports about Ti–Ag alloy.
The cells on the surfaces of Cu- or Ag-containing antibacterial titanium alloys exhibited a polygonal shape with a large number of filopodia and lamellipodia, indicating that the alloys exerted good cell spreading behavior [52,253,263,271]. Results from in vitro clearly showed antibacterial titanium alloys had no cytotoxicity (cell toxicity grade 0–1) to MG-63 cells [261,265,312], rBMSCs [52,253], L929 cells [273,285], MC3T3-E1 cells [73,263,270,271,313], V79 cells [17], NIH3T3 cells [285], as shown in Fig. 18.
Fig. 18.
The cell viability of antibacterial titanium alloys (data from Refs. [52,73,253,261,263,265,270,273,285,312]). Note: The cell viability was expressed by a relatively growth rate (RGR). RGR = 75%–100% is cell toxicity grade 1 and RGR≥100% is cell toxicity grade 0 (grade 0–1 means no cytotoxicity).
Zhang [285] indicated addition of Ag element would not reduce the biocompatibility of titanium alloy. Ti–Ag alloys with 5–20% Ag, no matter in the as-cast condition or the solid solution condition, showed low cell toxicity to L929 and NIH3T3 cell lines (less than Grade 1). In Zhang's study [312], Ti–Cu sintered alloys showed no cytotoxicity to MG63 cells, even containing as high as 25% Cu, had no effect on cell proliferation and differentiation, including cell adhesion and spread, which indicating Ti–Cu sintered alloys had very good cell biocompatibility. Ma's research [270] indicated Ti–6Al–4V–5Cu alloy had heat treatment under different conditions performed non-cytotoxicity (>90%) but heat treatment affected the cytotoxicity of Ti–6Al–4V–5Cu antibacterial titanium alloy. Increasing the solution temperature up to 1050 °C could precipitate more β phases carried more interstitial Cu atoms leading to easy to release more Cu, which in turn compromised the viability of MC3T3-E1 cells on Ti–6Al–4V–5Cu alloy. However, the aging treatment after solution treatment further increased cell activity.
Current studies have shown that compared with cp-Ti, Ti–Cu alloy promotes or has no effect on cell differentiation. It was reported the AKP value of the Ti–10Cu sample at day 1 was significantly higher than the value of the cp-Ti value (p < 0.01) [261]. While in another research [312], no difference in AKP value after incubation for 3 days or longer (p > 0.05) was found between cp-Ti sample and Ti–Cu alloy (2–25 wt% Cu), indicating that even Cu content up to 25 wt% did not bring any influence on the differentiation of MG63.
Moniri [265] discovered significant amounts of calcium and phosphorous deposited on Ti–Cu alloy compared to pure titanium, indicating that Ti–Cu alloy stimulated osteoblast formation and osseointegration due to the release of Cu2+ ions as well as to its nanostructural properties. Moreover, Ti-Cu [263] and Ti–Ag [285] alloy were reported to have good hemocompatibility with extremely low hemolysis rate (less than 1%) and less platelet adhesion.
7.5. In vivo test
Only Ti–Cu alloy has been carried on in vivo experiments, as summarized in Table 9. In vivo results showed that Ti–Cu alloy had strong anti-infection and anti-inflammatory activity [17,195], as shown in Fig. 19(a-e). In addition, the osteoid formations on sintered Ti–10Cu alloy was as good as pure titanium [251]. In Liu's study, heat-treated Ti–5Cu alloy effectively reduced the bone resorption and stimulated the new bone formation compared with pure titanium, as shown in Fig. 19(f) [255]. In addition, it was also reported the postoperative blood copper level was not significantly different from preoperative, indicating Ti–Cu alloy mainly had local effects and could be safe for the whole body [17].
Table 9.
In vivo results of antibacterial Ti–Cu alloy.
| Year | Ti–Cu alloy | Animal model | In vivo results | [Ref.] |
|---|---|---|---|---|
| 2009 | Ti–1Cu Ti–5Cu (No machining and treatment process) |
Japanese white rabbits | 1) Ti-1% Cu alloy significantly inhibited inflammation and infection. 2) The osteoid formations on Ti-1% Cu alloy was excellent as well as pure titanium, but was diminished in Ti-5% Cu alloy. 3) The postoperative blood copper level was not significantly different from preoperative. |
[17] |
| 2015 | Ti–10Cu (Powder metallurgy) | New Zealand white rabbits | Ti–10Cu alloy had as good bone biocompatibility as cp-Ti, which brought no toxicity to the surrounding cell and tissue and no delayed to bone healing. | [251] |
| 2018 | Ti–5Cu (850 °C/2 h/furnace cooling | Beagles | Ti–Cu implant not only effectively reduced the bone resorption but also stimulated the new bone formation compared to Ti. | [255] |
| 2019 | Ti–10Cu (Powder metallurgy) | New Zealand white rabbits | Ti–Cu alloy exhibited strong in vivo antibacterial properties while serious infection, inflammation and fester were observed in the cp-Ti. | [195] |
Fig. 19.
The in vivo results of copper-containing antibacterial titanium alloy. (a) Implantation of Ti–10Cu and cp-Ti implants with bacterial suspension and general observation of the implantation site after the implantation. (b)White blood cell (WBC) test results before and after implantation of Ti–10Cu and cp-Ti implants. (c)Typical photos of colonization by S. aureus taken from Ti–10Cu and cp-Ti implants which had been implanted with bacterial suspension in rabbit muscle. (d) Pathological photographs of the muscle surrounding the Ti–10Cu and cp-Ti implants. (f) Experimental design of Ti–5Cu and cp-Ti dental implants for in vivo animal tests and the radiographic analysis, Micro-CT, histological observations. (a–e): [195], copyright 2019, Elsevier. (f): [255], copyright 2018, Elsevier.
8. Antibacterial pure zinc and zinc alloys
Zn-based alloys have been turned focus towards an ideal degradation materials (such as cardiovascular stents) taking account of their moderate degradation rate in comparison with the fast biodegradation rate of Mg and Mg alloys and the low biodegradation rate of Fe-based materials due to different standard potentials of Mg, Zn and Fe (−2.372 V, −0.762 V and −0.440 V, respectively) [314]. However, the mechanical properties of Zn-based alloys are insufficient for most biomedical applications, and some antibacterial elements alloying may be effective to solve the problem as well as improve the infection problem.
8.1. Antibacterial properties
Pure Zn did not show obvious antibacterial activity both in the agar diffusion plate test and in the plate method [[314], [315], [316]]. Tang [314] indicated the reason why pure Zn had no obvious antibacterial property was attributed to its high corrosion resistance in c-SBF solution and low release of Zn ions. The antibacterial rates of extruded Zn against both E. coli and S. aureus reached at 75.49% and 72.36%, respectively [316], and the inhibition zone for S. aureus of extruded Zn was less than 1 mm [314]. But in other studies, Zn showed strong antibacterial function as the antibacterial rate was over 98.1% [317] and inhibition zone was about 6.19 mm [318]. Therefore, zinc alloys containing Cu, Mg or Ag etc. were designed to further improve the antibacterial property, as Table 10.
Table 10.
Antibacterial zinc and zinc alloys.
| Alloy system | Bacteria (antibacterial rate[Ref.]) |
|---|---|
| Pure Zn |
S. aureus (a 72.36%- >98.1% [316,317],b 0.0 ± 0.3 mm [314],b 6.19 ± 0.42 mm [318]); E. coli (a 5%- >98.1% [[315], [316], [317]]); Streptococcus gordonii (a about 64% for 12 h incubation [319]) Mixed oral bacteria (a about 65% for 12 h incubation [319]) |
| Zn-0.5Al | E. coli (b 1.9 ± 0.1 mm [320]) |
| Zn-0.8Mn | E. coli (a 81.3% [321]) |
| Zn-xCu (x = 1–4 wt%) |
S. aureus (a 81.86% [316],b 0.5 ± 0.4mm-3.5 ± 0.2 mm [314];c [322]); E. coli (a 88.77% [316]) |
| Zn-xAg (x = 1–4 wt%) |
E. coli (a 58.9%–100% [315]); S. gordoniic,d [323] |
| Zn-0.05 Mg | E. coli and S. aureus (a > 98.1% against both bacteria [317]) |
| Zn-0.8Mn-0.4Cu/Ag/Ca | E. coli (a 93.7%,a 91.8%,a 84.5%, respectively [321]) |
| Zn-xCu-yFe (x = 0.5, 3 wt%) |
S. aureus (a 90.17%–93.60% [316]); E. coli (a 92.99%–96.46% [316]) Streptococcus gordonii (a about 76% for 12 h incubation [319]) Mixed oral bacteria (a about 84% for 12 h incubation [319]) |
| Zn–1Cu-0.1Ti | S. aureus (b 6.19 ± 0.42 mm-6.99 ± 0.33 mm [318]) |
| Zn-0.5Al-xMg (x = 0.1–0.5 wt%) | E. coli (b 3.3 ± 0.2mm-6.3 ± 0.2 mm [320]) |
Note: 24 h incubation as default.
Measured by the plate count method.
Measured by the Agar diffusion plate test.
Measured by Live/Dead staining.
Measured by crystal violet staining.
Addition of Cu significantly improved the antibacterial property of pure Zn and the antibacterial property increased gradually as the Cu content increased because Cu ions have stronger antibacterial ability than Zn ions and the addition of Cu accelerated the degradation rate of Zn and increased the Zn release concentration [314,316,318]. The satisfied antibacterial properties of Zn–Cu was obtained at ≥ 2 wt% Cu [314]. Another research also confirmed that Zn–4Cu could effective inhibit bacteria adhesion and biofilm formation due to the antibacterial function of both Cu ion and Zn ion [322].
Alloying by Ag element can also enhance the antibacterial ability of Zn alloy in a content dependent way due to the strong and broad antibacterial function of Ag. Porous Zn–1Ag prepared by the air pressure infiltration method exhibited an antibacterial rate of 58.9% while porous Zn-3.5Ag was of nearly 100% antibacterial rate [315]. Zn–4Ag alloy was also reported to effectively inhibit bacterial adhesion and biofilm formation in comparison to Ti–6Al–4V alloy, demonstrating that adding Ag into pure zinc had a good antibacterial effect [323]. Zn-based with small amount of Cu or Ag (0.4%) also showed good antibacterial rate (>90%) against E. coli which was mainly due to the high Zn ion release [321].
Zn alloy with Mg as alloying element also exhibited good antibacterial activity. Zn-0.05 Mg alloy exhibited over 98.1% antibacterial function against both E. coli and S. aureus [317]. In another research, Zn–Al alloys with or without Mg both had antibacterial activity, while Zn–Al–Mg alloys indicated stronger inhibitive function against E. coli than Zn-0.5Al alloy [320].
The addition of Cu and Fe elements improved the antibacterial effect due to the high Cu ion and Zn ion release caused by fast degradation of Zn alloy. Zn–3Cu-0.2Fe and Zn–3Cu-0.5Fe alloys showed good antibacterial activity (>90% reduction) against both E. coli and S. aureus [316].
8.2. Mechanical properties
Zinc and zinc-based alloys are easier to cast and process due to their low melting points, low chemical reactivity and good machinability [324]. Similar to pure magnesium, the strength and ductility of pure Zn is poor, which could not meet the clinical requirements. The yield strength (YS) of pure Zn is from 10 MPa to 140 MPa, the ultimate tensile strength (UTS) is from 18 MPa to 180 MPa, the elongation is 0.32% to >60%, and the Vickers hardness is 25 HV to 44 HV [314,317,[324], [325], [326], [327], [328], [329], [330]], depending on the metal processing. Improvements in mechanical properties as well as antibacterial properties may be achieved by element alloying and appropriate thermomechanical treatment by principal solid solution and second-phase strengthening. Fig. 20 summaries the mechanical properties of some zinc-based alloys.
Fig. 20.
Mechanical properties of antibacterial zinc alloy (data from Refs. [314,317,[321], [322], [323],[325], [326], [327], [328], [329], [330]]). a) yield strength, b) ultimate tensile strength, c) elongation.
Addition of Cu significantly improved the strength and the elongation [314]. Cu has a relatively high solid solubility of about 1.53 wt% in Zn alloys, thus CuZn5 phase will precipitate after homogenized heat treatment when Cu content is higher than the critical [331]. CuZn5 phase could also contribute to grain refinement during subsequent hot extrusion. Therefore, the strengthening mechanism of Cu-containing zinc alloy could be attributed to CuZn5 precipitation hardening, as well as solid-solution strengthening of Cu element [318,322].
Interestingly, Mg has also been reported as an alloying element for Zn-based alloy [317,320,332,333]. The solid solubility of magnesium in zinc is low (0.15 wt%) and the Mg2Zn11 intermetallic phase forms in the structure even at small amounts of magnesium [326], thus the mechanical properties of Mg-containing zinc alloys can considerably be improved by the presence of fine and hard Mg2Zn11 intermetallic particles [329], but alloying with high magnesium concentrations would cause brittleness of such alloys due mainly to brittle eutectic phase [326]. It is proposed that optimal Mg concentration falls in between 0.01 wt% and 3 wt% [328]. Adding Mg element into Zn–Al alloy also improved the mechanical properties, which was attributed to that the presence of Mg2(Zn, Al)11 phase impeded the grain boundary motion and grain growth of ternary Zn–Al–Mg alloy [320]. In addition, Niu [322] considered adding trace Mg in Zn–Cu alloy had the potential as a biomaterial. Similar, adding the alloying elements such us Ca and Sr was also effective on the improvement of the mechanical property zinc alloys [321,330].
The metal preparation process and the metal forming process also have great influence on the mechanical properties. Extrusion, rolling, forging, annealing, etc. could improve its mechanical properties significantly [[334], [335], [336]]. It is widely accepted extrusion not only eliminates brittleness of the as-cast alloys but also significantly improves their strengths [318,321,328]. Extruded zinc alloy consisted of very refined Zn matrix, and the refined microstructure characteristics could be closely related to the high strength and ductility [317].
8.3. Corrosion properties
As a biodegradable biometallic alloy, Zn attracts much attention due to its moderate degradation rate in comparison with magnesium alloy and iron. However, it is still desired to accelerate the degradation rate of Zn to some extent. The corrosion rate of pure Zn in c-SBF solution was as low as 22.1 ± 4.7 μm/year, which is much lower compared with the reported Mg alloys and mainly be attributed to the higher standard potential of Zn compared with Mg-based alloys [314]. It was reported that the generated Zn(OH)2 and other corrosion products like ZnCO3 and Zn3(PO4)2 could form a compact surface layer, and thus restrained the degradation of Zn alloys [328]. Alloying elements have influence on the corrosion properties of Zn alloys dependent on the element and content. Table 11 lists the corrosion properties of some reported antibacterial zinc alloys.
Table 11.
The corrosion properties of antibacterial zinc alloys.
| Alloys | Condition | Solution | Ecorr (V) | icorr (μA/cm2) | Corrosion rate (μm/year) | Ref. |
|---|---|---|---|---|---|---|
| Zn–4Cu | as-cast+380 °C/9 h + extrusion | Hank's | _ | 4.1 ± 0.2 | 9.41 ± 1.34 | [322] |
| Zn–1Cu | as-cast+360 °C/8 h + extrusion | c-SBF | _ | _ | 33.0 ± 1.0 | [314] |
| Zn–2Cu | _ | _ | about 27 | |||
| Zn–3Cu | _ | _ | about 30 | |||
| Zn–4Cu | _ | _ | about 25 | |||
| Zn-2.5Ag | as-cast+410 °C/6 h and 12 h/W.C.+extruded at 250 °C | Hanks' modified solution | −1.12 ± 0.01 | 9.2 ± 0.9 | 137 ± 21 | [325] |
| Zn–5Ag | −1.12 ± 0.02 | 9.7 ± 0.7 | 144 ± 7 | |||
| Zn–7Ag | −1.14 ± 0.04 | 9.9 ± 0.6 | 147 ± 18 | |||
| Zn-0.5Al | as-cast | SBF | −1.074 ± 0.012 | 20.4 ± 1.3 | 150 ± 10 | [320] |
| Zn-0.5Al-0.1 Mg | −1.065 ± 0.013 | 17.3 ± 1.1 | 130 ± 10 | |||
| Zn-0.5Al-0.3 Mg | −1.034 ± 0.011 | 11.2 ± 0.7 | ||||
| Zn-0.5Al-0.5 Mg | −1.018 ± 0.012 | 9.5 ± 0.3 | 110 ± 10 | |||
| Zn-0.05 Mg | N.A | SBF | _ | _ | 728 | [317] |
| Zn-0.8Mn | As-cast | SBF | −1.070 ± 0.02 | 6.76 ± 0.35 | 101 ± 9 | [321] |
| Zn-0.8Mn-0.4Ag | −1.190 ± 0.01 | 11.22 ± 0.79 | 168 ± 21 | |||
| Zn-0.8Mn-0.4Cu | −1.180 ± 0.01 | 8.91 ± 0.67 | 133 ± 15 | |||
| Zn-0.8Mn-0.4Ca | −1.160 ± 0.01 | 10.72 ± 1.28 | 160 ± 19 | |||
| Zn–1Cu-0.1Ti | As-cast | Hanks' | −1.025 ± 0.264 | 21.5 ± 0.4 | 315 ± 6 | [318] |
| Zn–1Cu-0.1Ti | Hot-rolling | −1.123 ± 0.185 | 111.2 ± 0.9 | 1628 ± 13 | ||
| Zn–1Cu-0.1Ti | Hot-rolling + cold rolling | −1.100 ± 0.201 | 67.7 ± 0.5 | 991 ± 7 |
Cu and Ag have high standard electrode potential than Zn. The intermetallic phase between Cu–Zn or Ag–Zn normally acts as cathodic and enhances the corrosion of zinc matrix. The degradation rates of antibacterial Zn–Cu alloys showed a little increase compared with pure Zn due to galvanic corrosion between CuZn2 phase and matrix, but there was no significant difference among Zn–Cu alloys with different Cu contents, suggesting the standard potential of CuZn5 phase might be close to that of Zn matrix, and thus no severe galvanic corrosion occurred. On the other hand, fine and uniform corrosion pits may also confirm that severe galvanic corrosion did not occur [314]. The addition of Ag element clearly decreased the corrosion resistance of Zn–Ag alloy, which might be related to the formation of AgZn3 phase, inducing micro-galvanic corrosion, which finally leads to a decreased corrosion resistance of Zn–Ag alloys [315,323,325]. But in another research, addition of 0.4 wt% Cu/Ag/Ca accelerated corrosion of Zn-0.8Mn alloy, among which the effect of Ag was the most prominent [321].
It was reported the stable corrosion rate of Zn-0.05 Mg alloy immersed in the SBF slightly increased compared to that of Zn [317], but the addition of Mg had a positive effect on the corrosion resistance of Zn–Al alloys [320]. It was further reported that the corrosion resistance increased with increasing Mg content in Zn–Al–Mg alloys due to the homogenously dispersed Mg2(Zn, Al)11 secondary phase in the matrix, which leads to the formation of an uniform surface film of Mg-containing corrosion products, postpones the occurrence of localized corrosion and can effectively protect the Zn-based alloy [320].
Metal produce processing also significantly influences the corrosion rate of Zn alloy. In Lin's study [318], the as-cast Zn–Cu–Ti alloy showed the best corrosion resistance, and hot rolling deteriorated the corrosion resistance. It was thought that second phases of TiZn16 and ε-CuZn5 mainly distributed along the grain boundaries and formed a network in the matrix in the as-cast alloy and protected the alloy from fast corrosion. The hot-rolling broke the network and accelerated the corrosion by serious galvanic corrosion.
8.4. Biocompatibility
The stimulation for osteogenesis by zinc ion has been approved by numerous researches [337,338]. Generally, low concentrations of Zn2+ promoted viability, proliferation, adhesion, and migration, while high concentrations of Zn2+ were just the opposite [339]. The current standard for cytotoxicity evaluation is not suitable for biodegradable metals, and a minimal 6 times to a maximal 10 times dilution of extracts to better simulate the in vivo environment were recommended for the in vitro cytotoxicity evaluation of biodegradable Mg-based materials [340]. The dilution of extracts may also be applicable for biodegradable Zn-based materials though further study is required [314,322].
100% extracts of pure Zn exhibited a low cell viability to the EA. hy926 [314], MC3T3-E1 [318], and MG-63 cells [318], however, when the extract was diluted to a certain concentration, it had no toxicity to cell viability. The concentration of released metal ions higher than the ion threshold for cell proliferation may have adverse effects on cells, therefore, it is desirable to maintain a low concentration of Zn2+ in the local tissue by controlling the slow degradation rate of zinc alloy would be very crucial for the biological properties of antibacterial zinc alloy [318]. It is now recognized that adding appropriate amount of Ag, Cu and Mg to zinc showed good cytocompatibility, as shown in Table 12.
Table 12.
The cell viability of antibacterial pure zinc and zinc alloy.
| Alloy system | Metal or alloy | Cell | Cell viability (Cell culturing time) | [Ref.] |
|---|---|---|---|---|
| Pure Zn | Zn | EA.hy926 | 100% extract: cytotoxicity (1, 3, 5 days); 50% and 10% extracts: >90% (1, 3, 5 days) |
[314] |
| Zn | MC3T3-E1 | 100% extract: < 55% (1, 3, 5 days); 25% extract: 64.48% (1 day); 12.5% extract: > 90% (1, 3, 5 days) |
[318] | |
| MG-63 | 100% extract: < 55% (1, 3, 5 days); 25% extract: 87.30% (1 day); 12.5% extract: > 90% (1, 3, 5 days) |
[318] | ||
| Ag-containing | Zn–4Ag | L929 | 100% extract: < 40% (1 day); 33.3% extract: approximately 40% (1 day); 16.7% and 10% extracts: approached 100% (1 day) |
[323] |
| Saos-2 | 100% extract: < 40% (1 day); 33.3%, 16.7% and 10% extracts: approached 100% (1 day) |
[323] | ||
| Cu-containing | Zn-xCu (x = 1–4 wt%) | EA.hy926 | 100% extract: cytotoxicity (1, 3, 5 days); 50% and 10% extracts: >90% (1, 3, 5 days) |
[314] |
| Zn–4Cu | EA.hy926 | 100% extract: only reaches 10–20% (1, 3, 5 days); 50% and 10% extracts: approaching 100% (1, 3, 5 days) |
[322] | |
| Zn–3Cu–Fe | EA.hy926 | 100% extract: < 75% (3 days); 75% extract: 75–90% (3 days); 50% and 10% extracts: > 100% (3 days) |
[316] | |
| A7r5 | 75% and 100% extracts: < 75% (3 days); 50% extract: >75% (3 days); 10% extract: > 100% (3 days) |
[316] | ||
| Zn–1Cu-0.1Ti | MC3T3-E1 | 100% extract: < 55% (1, 3, 5 days); 25% extract: 82.74% (1 day); 12.5% extract: > 90% (1, 3, 5 days) |
[318] | |
| MG-63 | 100% extract: < 55% (1, 3, 5 days); 25% extract: 65.45% (1 day); 12.5% extract: > 90% (1, 3, 5 days) |
[318] | ||
| Mg-containing | Zn-0.8 Mg | U-2 OS | 75% and 100% extracts: < 20% (1 days); 50% extract: >80% (1 days); 10% extract: > approaching 100% (1 days) |
[327] |
| L929 | 50%, 75% and 100% extracts: < 20% (1 days); 25% extract: > 100% (1 days) |
[327] | ||
| Zn-0.5Al–Mg (0.1–0.5 wt%) | MC3T3-E1 | extraction medium: 102–108% (7 days) | [320] |
Research indicated addition of Ag into pure Zn would not lead to potential cytotoxicity, because the metabolic activities and the proliferation activities of L929 cells and Saos-2 cells always reached 100% when the extract of Zn–4Ag was diluted to only less than 33% and 16.7%, respectively [323].
In the study [314,322], the EA. hy926 cells showed good viability cultured in 50% and 10% extracts of Zn-xCu alloys (cell viability higher than 90%), indicating good cytocompatibility of Zn-xCu alloys. Pure Zn with Cu or Cu plus Fe could facilitate the growth of endothelial cells and promote the completion of endothelial cell function and inhibit the adhesion growth of smooth muscle cells [316]. Another research indicated the extract of Zn–1Cu-0.1Ti antibacterial alloy at a concentration ≤25% showed no significant cytotoxicity to MC3T3-E1 and MG-63 cells [318].
The 50% extract of Zn-0.8 Mg alloy exhibited acceptable cell viability U-2 OS cells(cell viability higher than 80%), while it needed to be diluted to 25% could make L929 cells viability reach 100% [327]. Addition of Mg to Zn-0.5Al alloy could improve the cells viability of MC3T3-E1 cells from 101 ± 1% to 108 ± 1% and the cells viability increase with increasing Mg content, possibly because Mg contributed to decreasing in the dissolution rate of Zn2+ and pH values in physiological environments [320].
In addition, zinc alloy exhibit lower hemolysis rates, less platelet adhesion, and adherent platelets remain ellipsoidal morphology without irreversible transformation, and the addition of Cu or Cu plus Fe element has no adverse effect on the hemocompatibility of pure Zn [316].
8.5. In vivo test
So far, limited data on in vivo experiments of antibacterial pure zinc and zinc alloy was reported, as summarized in Table 13. The results indicated that Zn and Zn-0.05 Mg alloy as vascular stent and bone implant performed well in vivo antibacterial activity and compatibility.
Table 13.
In vivo results of antibacterial pure zinc and zinc alloy.
| Year | Pure Zn and Zn alloy | Animal model (position) | Application | In vivo results | [Ref.] |
|---|---|---|---|---|---|
| 2015 | Zn | Sprague Dawley rats (abdominal aorta) | Endovascular stent | Zn did not promote restenosis responses and might suppress the activities of inflammatory and smooth muscle cells | [341] |
| 2017 | Zn | Japanese rabbits (abdominal aorta) | Endovascular stent | No severe inflammation, platelet aggregation, thrombosis formation or obvious intimal hyperplasia was observed after 12 months post implantation (as Fig. 21). | [342] |
| 2018 | Zn and Zn-0.05 Mg | Sprague Dawley rats (bilateral tibial shaft) | load-bearing implant | Zn alloy could promote the formation of new bone tissue at the interface between the implant material and bone tissue in vivo, and the degradation had no harm to the function and histology of important organs(as Fig. 22). | [317] |
9. Antibacterial pure magnesium and magnesium alloys
Magnesium-based materials were first introduced as orthopedic biomaterials in the first half of the last century [343]. Mg and Mg alloys as a new generation of biomaterial can eliminate the need for the second operation for implant removal due to their biodegradability, which has attracted much attention worldwide. The density and the elastic modulus of Mg and its alloys are more closer to human bone than others biometallic alloys [344]. It is worth noticing that Mg was firstly reported to possess antibacterial function due to the increase of pH value during its degradation in the bacterial solution, which is meaningful in biomedical application as the infections associated with surgical implants are currently becoming a serious issue [345]. However, they suffer from the “trilemma” problem of compromising among sufficiently high mechanical properties, good biocompatibility and proper degradation rate conforming to the growth rate of new bones. Fortunately, modifying alloy composition provides an approach for controlling the above problems. The antibacterial activity of many magnesium alloys has been investigated, including pure Mg, Ag-bearing magnesium alloy, Cu-bearing magnesium alloy as well as Zn-bearing magnesium alloy, etc.
9.1. Antibacterial properties
Mg showed an antibacterial activity that was comparable to a fluoroquinolone antibiotic against E. coli, P. aeruginosa and S. aureus [345]. A significant amount of researches also indicated Al-containing magnesium alloys such as AZ31, AZ91 and Re-containing magnesium alloys such as Mg–4Y showed strong antibacterial activity [[346], [347], [348]]. It was widely accepted that the antibacterial ability was mainly caused by the pH raise with the degradation of Mg metal in the incubation process [174,345,346].
However, the antibacterial effect of Mg might be insufficient in a dynamic environment like human body, because the degradation rate of pure Mg and magnesium alloys in vivo is not as fast as the rate in vitro and a high pH cannot be maintained to achieve long-term inhibition to bacteria [349,350]. In addition, some studies have shown that corrosion products formed a deposition layer on the surface of magnesium, which provides a favorable place for bacterial adhesion. Therefore, the antibacterial activity in vivo will be significantly reduced [351]. In vivo mouse model also indicated magnesium was effective against bacteria during in vitro assays or during surgery, but bacteria thrived in the presence of magnesium after implantation [352]. Therefore, magnesium alloys containing Ag, Zn or Cu etc. were designed as implant materials to combine the favorable properties of magnesium with the well-known antibacterial property of antibacterial elements [87,353,354]. Table 14 summaries some reported antibacterial pure magnesium and magnesium alloys in recent years.
Table 14.
Antibacterial pure Mg and Mg based alloys.
| Alloy system | Bacteria (antibacterial rate) |
|---|---|
| Pure Mg |
E. coli (>99.9% after 6 h [346], planktonic bacteria: 58.3% [355]/59.9% [356] and on the surface: 59.4% [355]/70.3% [356], <5% [72], No [345,347]); S. aureus (>99.9% after 6 h [346], 80% after 12 h and 100% after 24 h [357], 100% after 72 h in the environment of normal pH values and no antibacterial effect in the neutral environment [358], planktonic bacteria: 50.3% [355]/34.5% [356] and on the surface: 51% [355]/60.7% [356], both in vitro and in vivo [351], No [345,352]); P. aeruginosa (both in vitro and in vivo [352], No [345]); MRSA (planktonic bacteria: 68.9% and on the surface: 62.8% [350], in vivo [72,350], <10% [72], No [359]); C. albicans (>98% [346], about 90% [353]) S. epidermidis(planktonic bacteria: 50.8% [355]/37.7% [356] and on the surface: 49.8% [355]/56.8% [356], <5% [72]) S. aureus: S. epidermidis = 1:1 (49.7% after 15 h [360]) |
| AZ31 |
S. aureus (>99.9% after 6 h [346]); E. coli (>99.9% after 6 h [346], No [347]) |
| AZ91 | A. baumannii (>99.9% after 18 h in vitro, in vivo [361]); |
| Mg–4Y | E.coli [347] |
| Mg–Ga |
E. coli (planktonic bacteria: 51.8%; on the surface: 67.8%) [356]; S. aureus (planktonic bacteria: 48.3%; on the surface: 63.0%) [356]; S. epidermidis (planktonic bacteria: 46.5%; on the surface: 63.9%) [356] |
| Mg-xCu |
S. aureus (100% [362], 100% after 12 h [353], 100% after 72 h both in the normal pH values and neutral environment [358]); E. coli (about 33%- > 90%), S. epidermids, and MRSA (>90%) [72]; C. albicans (>99.9% [353]) |
| Mg-xAg | S. aureus: S. epidermidis = 1:1 (72.5%–83.5% after 15 h [87], 81.4%->95% after 15 h [360]); |
| Mg–3Zn | E. coli and S. aureus [363,364] |
| Mg-xSr |
E.coli (planktonic bacteria: 48.0% and on the surface: 63.5% [356]); S. aureus (80% after 12 h and 100% after 24 h [357], planktonic bacteria: 29.1% and on the surface: 59.1% [356]) S.epidermidis (planktonic bacteria: 35.6% and on the surface: 58.7% [356]) |
| Mg–3Zn-xAg | E. coli and S. aureus [363,364] |
| Mg–2Zn-0.5Ca | MRSA [359] |
| Mg–4Zn–1Sr | MRSA [359] |
| Mg–Ca–Sr |
E. coli (planktonic bacteria: 53.1%, on the surface:66.5% [356]); S. aureus (>90% [354], planktonic bacteria: 40.9%, on the surface: 64.0% [356]); S. epidermidis (planktonic bacteria:44.9%; on the surface: 68.0%) [356] |
| Mg–1Ca-0.5Sr-xZn | S. aureus (76.9%- > 96.6%) [354] |
| Mg-Nd-Zn-Zr |
E. coli (planktonic bacteria: 59.4%–83.1%; on the surface: 74.8%–94.8%) [355]; S. aureus (planktonic bacteria: 51%–79.1%; on the surface: 74.2%–88.4%) [355]; S. epidermidis (planktonic bacteria:49.8%–79.8%; on the surface:69.5%–89.5%) [355] |
| Mg–Zn–Y-Nd-xAg | E. coli and S. aureus (both > 99.9% [365], No [19]) |
| Mg–Zn–Y-Nd-xCu | E. coli and S. aureus (both > 99.9%) [366] |
| Mg–Ca–Mn–Zn-xAg | E. coli and S. aureus [367] |
Note: 24 h incubation on the surface as default.
“No” means no antibacterial rate is shown in the literature.
Mg–Cu alloys possessed excellent and prolonged antibacterial effects against E. coli because of the high pH value as well as sustain release of Cu ion (50–100 ppb after 24 h and 100–200 ppb after 72 h [358]. Addition of Cu above 0.1 wt% could efficiently enhance the antibacterial activity against C. albicans (almost 100% reduction) [353] as well as S. aureus [362]. Similar, the extruded Mg–Zn–Y-Nd-xCu alloy with at least 0.1 wt% Cu could ensure the alloy had an antibacterial rate of more than 99% against E. coli and S. aureus [366]. Li [72] further confirmed the in vivo antibacterial activity of Mg–Cu alloys in a rabbit model of chronic tibial osteomyelitis and measurement with imaging examination, microbiological cultures.
Mg–Ag alloys (2–6% Ag) at solution treatment demonstrated higher antibacterial activity than pure Mg [87]. In addition, extrusion treatment without solution treatment would optimize antibacterial properties of Mg–Ag alloys [360]. Zhan et al. [363] found that the implantation of new magnesium alloy materials, Mg–3Zn, Mg–3Zn–1Ag and Mg–3Zn–3Ag, showed obvious antibacterial effect on S. aureus or E. coli infection, and the addition of silver in magnesium alloy could enhance its antibacterial ability. Li [365] also proved that the antibacterial rate of Mg–Zn–Y-Nd-xAg alloy increased with the increase of Ag content, while Mg–Zn–Y-Nd alloy did not have antibacterial property.
Mg-Nd-Zn-Zr displayed a similar but higher antibacterial activity than pure magnesium, which was caused by the combination or synergy of increased pH value with the degradation and the antibacterial function of both Zn and Zr element [355]. In another research, Zn-containing magnesium alloys Mg–1Ca-0.5Sr-xZn (x = 0, 2, 4, 6) contained at least 4% Zn to insure high antibacterial property (>96.6% reduction) and the good antibacterial property of the alloy was attributed to the antibacterial efficiency of both Sr ion and Zn ion [354].
In addition, it was also pointed out that the addition of trace Ga and Sr (0.1 wt%) could improve the antibacterial activity of pure Mg [356]. Mg-0.1Ga, Mg-0.1Sr and Mg-0.1Ga-0.1Sr alloy induced a less pronounced increase in pH but stronger toxicity to stains than pure Mg, as such the latter could be attributed to the release of Ga3+ and Sr2+ ions [356].
9.2. Mechanical and corrosion properties
Pure magnesium is extremely susceptible to corrosion, leading to losses of strength and toughness, which limits their biomedical application. While element alloying is an effective measurement to improve both the corrosion resistance and mechanical properties of magnesium [368]. The most common biomedical magnesium alloys contain aluminum and/or rare earth (RE) elements and it is known that Al and Re additions could improve the mechanical properties and corrosion resistance of Mg alloys [163].
As-cast Mg–Ag alloys with 2%–6% Ag continuously enhanced mechanical properties including hardness and strength meanwhile continuously reduced the corrosion resistance against DMEM and DMEM with FBS, which was attributed to the increase of Mg4Ag phases and dendrite structure [369]. In Tie's another study [87], heat treatment, including solution treatment and aging treatment, could significantly improve both mechanical properties and corrosion resistance of the as-cast Ti–Ag, and the heat-treated Mg–Ag alloys showed a significant better mechanical properties and lower corrosion rate than pure magnesium. Feng [19] suggested both the increase of Ag content and extrusion process improved the microhardness of magnesium alloys. Moreover, the degradation rate could be reduced by eliminating the precipitates in Mg–Ag alloys via solid solution treatment after extrusion process [360]. Li [365] found that a new phase, Ag51Nd14, was formed in the as-cast Ag-containing Mg–Zn–Y-Nd alloy. Due to the potential difference between the magnesium matrix and Ag51Nd14, a primary cell was formed between the magnesium matrix and Ag51Nd14, which increased the corrosion rate of the alloy. At the same time, the microhardness, tensile strength and compressive strength all showed an increasing tendency. Another study has shown addition of Ag (0.5–3% Ag) improved the microhardness of Mg–3Zn alloys, while only addition of 0.5% Ag improved the compressive strength and elongation of Mg–3Zn, and the addition of Ag content was not conducive to the corrosion performance [364].
The addition of Cu into as-cast magnesium alloy forming Mg2Cu intermetallic phases increased the hardness and strength while significantly accelerated the degradation rate of magnesium in the physiological environment due to galvanic corrosion between Mg matrix and Mg2Cu intermetallic phase [72,358]. Chen [353] indicated that finely and continuously distributed second phase could more effectively retard the corrosion when the content of Cu was low, while large and thick Mg2Cu intermetallic formed along the grain boundaries accelerated the corrosion when the content of Cu was high. For instance, the corrosion rate of Mg-0.3Cu alloy was almost 10 times faster than that of Mg-0.1Cu alloy. Yan [362] found that the solution treatment after extrusion of low Cu-containing magnesium alloy eliminated the second phase and reduced the grain size significantly by recrystallization.
Zn is known to be a good solid solution and precipitation strengthening agent in Mg alloys and thereby to be one of the most commonly used alloying elements in Mg [370,371]. In addition, zinc is able to elevate the corrosion potential of magnesium alloys, and thus reduce the corrosion rate [372]. Cai [368] indicated the tensile properties and corrosion properties of Mg–Zn alloys strongly depended on the volume fraction and existence form of secondary phases. Solution treatment enhanced corrosion resistance while aging treatment decreased the corrosion resistance [373]. In Qin's study [355], element alloying with Zn, Zr and Nd dramatically improved the corrosion resistance of pure Mg and the novel degradable as-cast Mg-Nd-Zn-Zr alloy has been proven to possess proper mechanical properties and enhanced corrosion resistance. Brar [163] reported that the increasing of Zn content (2–6% Zn) increased the corrosion rate of aging-treated Mg–Zn-0.5Sr.
Furthermore, adding a small amount of Sr and Ca could improve the mechanical properties of pure magnesium or magnesium alloy by the grain refinement mechanism, while decreased the corrosion resistance to generate a more rapid degradation, potentially because more second phase precipitates enhanced micro-galvanic corrosion [163,374]. But Berglund [375] further found that low amounts of alloying elements enhanced the corrosion resistance properties of as-cast Mg-xCa-ySr system (x = 0.5–7.0 wt%, y = 0.5–3.5 wt %), with an optimal composition of 1% Ca and 0.5% Sr.
9.3. Biocompatibility
Good bone tissue response was observed around pure Mg implant in comparison with cp-Ti implant, such as high bone mineral content (BMC) and bone mineral density (BMD) and low porosity [351]. On the other hand, the raise of pH due to magnesium alloy degradation caused hemolysis and cell toxicity [376]. Zhen [376] indicated when the pH value was higher than 10.3, the hemolysis was higher than 5% (the recommended value by standard), and when the pH value was 10.72, the hemolysis was as high as 47.77%. Zhen's results [376] also confirmed that severe death of L929 cell line was induced when pH raised over 9. It was reported the cytotoxicity of Mg was ascribed to high alkalinity and Mg2+ concentration caused by its high degradation rate, thereby adversely affecting cell adhesion and growth [376]. It is unrealistic for pure magnesium to rely on a high pH to achieve antibacterial effect, even if without cytotoxicity, because high osmolality or magnesium ion concentration causes osmotic shock in human cells [377], while alloying of antibacterial elements can improve the biocompatibility of magnesium without compromising antimicrobial properties.
It was reported human primary osteoblasts could survive on the surface of extruded Mg–6Ag alloy and solution-treated Mg–8Ag alloy but died on extruded Mg–8Ag alloy (without solution treatment), indicating that addition of proper Ag content and solution treatment improved the cytocompatibility of magnesium alloy [360]. In another research [87], Mg–Ag alloys after solution treatment were non-cytotoxic to MG-63 and RAW 264.7 (survival rates between 95% and nearly 100%) by a long-term cytotoxicity and cell adhesion test over 14 days, and lower release silver ion concentrations than LC50 (lethal concentration LC50, where 50% of the cells survive) also verified this point of view. Bakhsheshi-Rad [367] confirmed that Mg–Ca–Mn–Zn-xAg with 0–0.5 wt% Ag was more favorable for the cell viability and cell adhesion of MG-63 cells than that with 1–2.0 wt% Ag.
The release of bioactive Mg and Cu ions into the biological environment during the degradation of the as-cast Mg–Cu alloys could induce osteogenesis and stimulate angiogenesis without cytotoxicity towards HUVECs and MC3T3-E1 cells [358]. In the report [72], the as-cast Mg–Cu alloys were non-cytotoxic towards Balb/c 3T3 cells compared with cp-Ti. Similarly, the cytotoxicity towards rBMSC cells of Mg-0.1Cu alloy is grade 0 [362].
Addition of Nd, Zn and Zr improved the cytocompatibility towards hBMSC cells of magnesium but the alloy still had lower cytocompatibility than cp-Ti, which may be associated with the different pH value (the corresponding pH value of the medium was 7.26 for Ti, 9.08 for Mg and 8.24 for Mg-Nd-Zn-Zr [355]. He [354] also indicated that addition of Zn into Mg–1Ca-0.5Sr alloy exhibited a better biocompatibility with no obvious toxicity to MC3T3-E1 cells (cytotoxicity grade 0–1).
9.4. In vivo test
Three kinds of biodegradable Mg implants have been already approved by Conformite Europeene (CE) or Korea Food and Drug Administration (KFDA) [378]. It is worth mentioning that more and more Chinese surgeons and researchers are trying a lot in the research and development of antibacterial Mg based implants to accelerate clinical application, as listed in Table 15.
Table 15.
In vivo results of antibacterial pure magnesium and magnesium alloy.
| Year | Metal and alloy | Animal model (position) | Co-cultured with bacterial | In vivo results | [Ref.] |
|---|---|---|---|---|---|
| 2014 | Mg | Sprague Dawley rats (right femur cavities) | Mg implants precultured with MRSA (106 CFU/mL) with an OD600 of 0.1 for 24 h |
|
[351] |
| 2015 | Mg | Balb/c mice (lower tail artery) | Mg implants precultured in a 1 mL suspension of P. aeruginosa culture with an OD600 of 0.1 for 5 min. | Mg implants showed an enhanced resistance to bacterial colonization. | [352] |
| 2015 | Mg–Nb–Zn–Zr | Sprague Dawley rats | S. aureus in a concentration of 105 CFU/mL was injected into the implant position. |
|
[355] |
| 2016 | Mg | Sprague Dawley rats (femoral medullary cavity) | 50 μl bacterial suspension of MRSA was injected into the implant position. |
|
[350] |
| 2016 | Mg | murine | 5 μl bacterial suspension was injected into the implant position. | Bacterial agglomerates could be detected on the implant surface and peri-implant tissue. | [379] |
| 2016 | Mg-0.25Cu | New Zealand White rabbits (proximal tibial metaphysis) | 0.15 mL of 5% morrhuate sodium was injected into the implant position. |
|
[72] |
| 2018 | AZ91 | Long-Evans rats (humeral head) | 105 CFU (0.1 cc) of A. baumannii was injected into the implant position. | No significant differences in the antibacterial activity from the cp-Ti and AZ91 implants after a week implantation. | [361] |
The in vivo experiments results suggested even if Mg implants were pre-cultured with bacterial suspension, Mg implantation could effectively protect bone and surrounding tissues from infection, and inhibit the growth of bacterial on the surface of Mg and bone tissue [351,352]. However, Mg implants exhibited insufficient antibacterial effect in vivo against MRSA compared to the high antibacterial rate in vitro [350,379]. Bacteria could be detected on the surface of the Mg implant surface as well as peri-implant tissue. Moreover, inflammatory cells filled cavities in the cortical bone of the femur after 2 and 4 weeks of implantation [350]. Similarly, AZ91 magnesium alloy was not able to maintain its antimicrobial effects during a one-week implantation period in a rat humeral head [361].
Mg alloys with proper number of alloying elements effectively enhanced antimicrobial properties and biocompatibility of Mg. For example, a novel Mg-Nd-Zn-Zr magnesium alloy showed enhanced the antibacterial activity in vivo compared with cp-Ti and Mg, moreover, the alloy had a small amount of gas in the medullary cavity, mild medullary cavity swelling deformation and unconspicuous reconstruction of cortical bone compared with Mg [355]. Li [72] designed a series of Mg–Cu alloys that combine the favorable properties of Mg with the antibacterial properties of Cu and the in vivo results, as Fig. 23, indicated Mg-0.25Cu alloy reduced MRSA-induced osteomyelitis infection compared with Ti. On the other hand, the Mg-0.25Cu alloy induced no local or systemic side effects and deposited no obvious Cu2+ or Mg2+ ion complexes in the organs or tissues.
Fig. 21.
The in vivo results of pure zinc as endovascular stent [342], copyright 2017, Elsevier Ltd. (a) Selected 2D and 3D micro-CT images of zinc stents after 0, 1, 6 and 12 months implantation. Each time point is composed of three images: the left one is a 3D reconstruction of the zinc stent, among which the white one is the residue zinc stent and the green one represents corrosion products. (b) Corrosion morphologies of zinc stents after 1 and 12 months in vivo. (c) Representative low- and high-magnification photomicrographs of hematoxylin-eosin (H&E) stained sections of abdominal aorta after 1, 3, 6, and 12 months implantation of zinc stents. The asterisk marked region at 12 months indicates the neointima without stent struts left. (d) Angiography of rabbit abdominal aorta after 12 months implantation of zinc stents. The stented segments are marked by red arrows. (e) Lumen diameter and (f) diameter stenosis of stented segments after 1, 3, 6 and 12 months implantation calculated from quantitative vessel angiography (QVA) measurements. (g) Representative immunofluorescence staining images of macrophage antibody after 3 days, 1, 3, 6 and 12 months implantation and the number of macrophages per strut at each time point. (h) Representative low- and high-magnification SEM images of endothelial coverage on zinc stents. (i) Immunohistochemical staining images of CD31 antibody and α-SMA antibody, Masson's trichrome and Verhoeffe-Van Gieson (VVG) staining images.
Fig. 22.
The in vivo results of Zn and Zn-0.05 Mg alloy as bone implant [317], copyright 2018, Elsevier Ltd. (Ⅰ) Plain anteroposterior radiographs of pure Zn and Zn-0.05 Mg pins implanted in femoral shafts of rabbits. (Ⅱ) Blood biochemical indicators: (a) Albumin (b) alkaline phosphatase (c) ALT (d) AST and (e) CREA (f) UREA (g) serum magnesium (h) serum zinc. The black dot lines in (a)–(h) represent the health reference ranges. (Ⅲ) (a)–(d): Representative histology of cross-sections of bone-implant interface stained with Toluidine blue and observed by microscopy. There are normal cortical bones (blue arrow); implant (white arrow); newly formed bone fractions around the implant (red arrow); bone junction between cortical bone and new bone formation (green arrow). (e)–(h): element mapping analysis on a cross-section of bone/implant interface. Concentration of Zn (blue area); concentration of C (green area), medullary cavity regions and surrounding tissue; concentration of Ca (red area). Implants with a high Zn concentration, the newly formed bone fractions around the implants showing enriched Ca.
Fig. 23.
The in vivo results of Mg-0.25Cu [72], copyright 2016, Elsevier Ltd. Digital X-ray and MR images, surface topography photographs, hematoxylin-eosin (HE) staining, Giemsa staining of longitudinal sections, radiographic and histological scores of the left tibia of the healthy control group, 4 weeks after osteomyelitis induction group, and 4 weeks after implantation of Ti and Mg-0.25Cu intramedullary nails group.
10. Antibacterial cobalt alloys
Co-based alloys have been widely used in hip implants and knee implants due to their excellent wear resistance and corrosion resistance, and in stents due to their high stiffness and ductility and excellent corrosion resistance. Implant related infection or inflammation is still a serious clinical complaint.
10.1. Antibacterial properties
The development of antibacterial cobalt alloys mainly focuses on Cu-containing Co-based alloy, as shown in Table 16. It was reported that the addition of Cu did provide cobalt alloy with antibacterial property and the antibacterial ability of cobalt alloy was Cu content dependent. In the study of antibacterial performance of CoCrW–Cu alloy against E. coli, it was clear that much less bacterial colonies were observed in the group of CoCrW–Cu alloy compared with the group of CoCrW matrix alloy [21]. Meanwhile, when 2.13 wt% Cu was added into Co19Cr10Ni14W, the antibacterial rates against E. coli and S. aureus were only 63.3% and 51.1%, respectively. With the increasing of the Cu content to 4.39 wt%, the antibacterial rate sharply increased to >90% [380]. In another study, it was shown that 1 wt% Cu provided very good antibacterial property (94.2% reduction) for Co29Cr6Mo, and further increasing in Cu content enhanced the antibacterial property, 98.5% and 99.6% reduction at 2 wt% and 4 wt% Cu, respectively [20]. Ag element has also been added into Co–Cr alloy to fabricate antibacterial Co–Cr–Ag alloy. Ag significantly enhanced the antibacterial activity of Co–Cr alloys, with antibacterial rates of 90.5% and 72.6% against E. coli and S. aureus, respectively [381].
Table 16.
Antibacterial Co-based alloys.
| Alloy system | Bacteria (antibacterial rate[Ref.]) |
|---|---|
| Co–Cr–Mo | S. aureus, S. epidermidis, CNS (No [382]) |
| Co29Cr6Mo-xCu | S. aureus (>90% [20]) |
| Co(28–29)Cr9W-xCu |
S. aureus (>99.99% [383]); E. coli (>90% [21,383], No [384]) |
| Co19Cr10Ni14W-xCu | S. aureus (51.1%- > 90% [380]); E.coli (63.3%- > 90% [380]) |
| Co-30at.%Cr-5at.%Ag | S. aureus (72.6% [381]), E.coli (90.5% [381]) |
Note: 24 h incubation as default.
10.2. Mechanical and corrosion properties
The addition of Cu element could increase the antibacterial property, but also have an influence on the mechanical and corrosion properties. Cu element has very high solubility in Co matrix at high temperature but low solid solubility at room temperature, about 19.7 at% at 1367 °C and nearly 0% at room temperature, which means that the solid solution strengthening ability of Cu element in Co-based alloy is very week [20]. In the research, the hardness and strength of Cu-rich phase is lower than that of Co-matrix, which suggests that the dispersion strengthening ability of Cu-rich phase would be very low. Due to these two main reasons, the addition of Cu element and the precipitation of Cu-rich during the process of Cu-containing Co-based alloy do not contribute to the improvement of mechanical properties of Co-based alloys. In the research [20], the addition of Cu reduced the microhardness of Co–Cr–Mo alloy in a content dependent way, and thus might reduce the wear resistance. The tensile strength of Co–Cr–Mo–Cu alloy decreased with the addition of Cu element up to 2 wt% and then increased with further increasing of Cu content, but the elongation was improved by the addition of Cu element [20], as listed in Table 17. It was reported that the addition of 3% Cu reduced the bonding strength between Co–Cr–W–Cu alloy and porcelain in comparison with Co–Cr–W system, but the bonding strength between Co–Cr–W–Cu alloy and porcelain was still higher than the recommended value in Standard ISO 9693-1999 [385]. The research on CoCrWNi–Cu alloy also indicated that the addition of Cu slightly reduced the microhardness but had a limited influence on the compression properties of CoCrWNi alloy [386].
Table 17.
Microhardness and tensile properties of Co29Cr6Mo-xCu alloys [20].
| Alloys | Microhardness (HV) | Tensile strength (MPa) |
Elongation (%) | |
|---|---|---|---|---|
| 0.2% YS | UTS | |||
| Co29Cr6Mo–0Cu | 298.33 ± 29.41 | 423.61 ± 18.21 | 576.92 ± 50.36 | 10.7 ± 1.8 |
| Co29Cr6Mo–1Cu | 292.69 ± 28.63 | 310.38 ± 41.43 | 447.83 ± 24.85 | 18.5 ± 3.7 |
| Co29Cr6Mo–2Cu | 279.67 ± 26.79 | 432.73 ± 12.86 | 499.13 ± 21.65 | 11.5 ± 3.8 |
| Co29Cr6Mo–4Cu | 274.64 ± 26.05 | 440.53 ± 36.47 | 620.56 ± 15.24 | 15.2 ± 0.1 |
One of advantages of Co-based alloys as a biomedical material is their high corrosion resistance against body fluids. However, the addition of Cu slightly increases the corrosion current density of CoCrWNi–Cu in a Cu content dependent mode. For example, the corrosion current densities of CoCrWNi base alloy, CoCrWNi-2.13Cu and CoCrWNi-4.39Cu were 5.39 × 10−7 A/cm2, 6.59 × 10−7 A/cm2 and 1.02 × 10−6 A/cm2, respectively [380]. It was thought that the Cu-rich precipitate acted as a cathodic center, thereby enhancing the dissolution of the matrix. Another investigation on the change in the corrosion property of as-cast CoCrWNi–Cu alloy proved that the addition of Cu slightly reduced the corrosion resistance as a result of the galvanic corrosion caused by dendritic segregation [386]. Research on SLM Co–Cr–Cu alloy also indicated that the influence of Cu on the electrochemical data of SLM Co–Cr alloy in 0.9% NaCl was negligible [383]. It must be pointed out the change in the corrosion properties of Co-based due to the addition of Cu element slightly decreased the corrosion resistance but the difference was very limited [20]. Co-based with and without Cu element still keep a very low corrosion rate in simulated body fluid.
The addition of Cu also has influence on the martensite transformation [387], which in turn affect the final deformation behavior. Co-based alloy is normally subjected to hot deformation to refine the microstructure and improve the mechanical properties. The hot deformation behavior is closely related to the microstructure and the phase constitute. In Co-based alloy, fcc γ-Co phase provides very good ductility and deformability while hcp ε-Co phase shows low ductility and deformability. The addition of Cu element effectively inhibited the martensite transformation from γ-Co to hcp ε-Co phase, thus, Cu containing alloys showed good ductility and also good thermal deformation ability [20].
10.3. Biocompatibility
Cobalt (Co) is an essential trace element for human and an important component of cyanocobalamin. As we all know, cyanocobalamin is an essential vitamin (vitamin B12) which is required for the production of red blood (RBC) cells and the prevention of pernicious anemia [388]. Co was historically used to treat certain types of anemia as a result of its ability to stimulate hemoglobin and RBC production [389]. What should be noted is the recommended daily intake of Vitamin B12 in the US is 2.4 mg/d, corresponding to approximately 0.10 mg of Co [389]. There is ample evidence to indicate that Co exposures in highly elevated blood Co concentrations (>300 μg/L) can lead to certain endocrine, hematological, cardiovascular, and neurological effects in some animals and/or humans [390]. Human subchronic and chronic oral exposure to Co has been closely linked with effects on the hematological, thyroid and cardiovascular systems. The average measured Co blood concentrations in these cohorts ranged from 2.1 to 26 μg/L [389]. Pettersson [391] evaluated the cytotoxic effect and the inflammatory response of macrophages to Ti, Co Cr and Mo in a range of 0–800 μM. Cytotoxic effects was reported for all ions except Cr, but no differences in the release of IL-1β was found with exposure to Co and Cr ions. However, no detail data was available in this reference.
In the antibacterial CoCr alloy system, the addition of Cu and Ag could enhance antibacterial property, but also affect cytotoxicity of alloy. The effect of Cu on cytotoxicity of CoCrW alloy is in a Cu-content dependent feature. There was no cytotoxicity for Cu-bearing CoCrW alloys when the Cu content was less than 4 wt% [384]. In the study of ultrafine-grained Co-30at.%Cr-5at.%Ag alloy, the addition of Ag also significantly enhanced the antibacterial activity of Co–Cr alloys but showed no cytotoxicity [381].
11. Antibacterial pure copper and copper alloys
Copper has been registered at the U.S. Environmental Protection Agency as the first solid antibacterial material [392]. It is suggested pure copper has excellent antibacterial properties against S. aureus, E. coli, K. pneumoniae, P. aeruginosa, S. typhimurium, L. monocytogenes, Streptococcus sp. BY1, Enterococcus sp. BY2 and B. cereus BY3, etc. [[393], [394], [395], [396]]. Moreover, Yousuf [396] suggested the Gram-negative cells were more resistant than the Gram-positive cells. The high antibacterial property was anticipated as that copper ions are able to inhibit the survival of several microorganisms [[112], [113], [114], [115]], many also ascribed it to the mechanistic of contact killing bacteria [392]. However, the poor corrosion resistance and strength made pure copper itself unsuitable for use as biomedical implant even though it has such strong antibacterial properties. Some antibacterial pure copper or copper alloys reported in recent years as illustrated in Table 18, including pure copper, brasses, bronzes, copper-nickels and copper-nickel-zinc alloys.
Table 18.
Antibacterial Cu based alloys.
| Alloy system | Bacteria | Reference |
|---|---|---|
| Cu |
E. coli; S. aureus; MRSA; L. monocytogenes; M. tuberculosis strain R432 and R267; C. albicans; K. pneumoniae; P. aeruginosa; A. baumannii; Salmonella; Enteritidis; S. Typhimurium DT193 S9, DT120 S19 and DT66S20; S. typhimurium; Streptococcus sp. BY1; Enterococcus sp. BY2; B. cereus BY3 |
[110,111,[393], [394], [395], [396]] |
| Cu-xZn |
E. coli; L. monocytogenes; MRSA; C. albicans; K. pneumoniae; P. aeruginosa; A. baumannii; M. tuberculosis strain R432 and R267; Salmonella; Enteritidis; S. Typhimurium DT193 S9, DT120 S19 and DT66S20 |
[84,110,[393], [394], [395]] |
| Cu–Ni | E. coli; Salmonella; Enteritidis; S. Typhimurium DT193 S9, DT120 S19 and DT66S20 | [84,393] |
| Cu–5Sn | E. coli | [84,393] |
| Cu–3Si | L. monocytogenes | [394] |
| Cu–Ni–Zn |
E. coli; L. monocytogenes; MRSA; C. albicans; K. pneumoniae; P. aeruginosa; A. baumannii; M. tuberculosis strain R432 and R267 Salmonella; Enteritidis; S. Typhimurium DT193 S9, DT120 S19 and DT66S20 |
[84,[393], [394], [395]] |
| Cu–15Ni–8Sn | E. coli | [393] |
| Cu–2Ni–8Al | E. coli | [393] |
| Cu–10Ni-1.4Fe | L. monocytogenes | [394] |
| Cu–3Al–2Si | E. coli | [393] |
| Cu–1Mn–2Si | E. coli | [393] |
| Cu–23Sn–3Al | E. coli | [393] |
| Cu-22.5Zn-2.5 Pb |
C. albicans; K. pneumoniae; P. aeruginosa; A. baumannii; MRSA; S. aureus; M. tuberculosis strain R432 and R267 |
[395] |
| Cu–Zn–Sn–Pb | E. coli | [111] |
| Cu–Zn–Sn–Al |
E. coli; P. stewartii; A. johnsonii; S. panni; P. oleovorans; S. haemolyticus; S. epidermidis; S. warnerii; B. conglomeratum; M. luteus; K. marina; K. palustris |
[393,401] |
| Cu-2.8Al-1.8Si-0.4Co | L. monocytogenes | [394] |
It was reported the brasses were harder and more durable than copper, Cu–Ni and Cu–Ni–Zn had much greater corrosion resistance than copper and all of these alloys showed excellent antibacterial activity at room temperatures (20 °C) [393]. Zhu [84] revealed that the antimicrobial activity of copper alloys against S. enterica and S. Typhimurium increased as the copper content (60–99.9 wt% Cu) of the alloys increased, even the copper resistant strains only survived for short periods of time on the high copper content alloys. Pure Cu demonstrated both growth inhibition of 98% and 88% against two M. tuberculosis strains, R267 and R432, respectively, while the other copper alloys with >55 wt% Cu also showed effective inhibition effect against both nosocomial pathogens and M. tuberculosis [395]. In Yousuf's study [396], the cells exposed to rougher surface of rolled-out copper plates were killed more effectively and faster due to the faster Cu ion release from rougher surface.
Touch surfaces in hospitals, such as door handles, touch plates, bed rails, call buttons, toilet seats, etc., can be highly contaminated with microbes. Thus in several clinical studies, copper has been evaluated for use on touch surfaces, attempting to curb nosocomial infections [392,397,398]. Palza's clinical trial results [399] firstly showed that it was a viable route to add copper to organic materials for applying the antimicrobial effects of copper, such as plastic waiting room chairs with embedded copper metal nanoparticles, and metal hospital IV pools coated with an organic paint with nanostructured zeolite/copper particles. Borkow [400] even imagined possible future applications of Cu alloy include filtration devices capable of deactivating viruses in solutions, such as contaminated blood products and breast milk.
12. Antibacterial metallic glasses
The emergence of bulk metallic glasses (BMGs) offers a new opportunity to achieve biomaterials with the desirable traits of excellent mechanical, chemical properties, process ability as well as low cytotoxicity, which make them as potential candidates for medical implants [[402], [403], [404], [405]]. Fe-based, Ni-based and Cu-based metallic glasses were firstly reported in 2012 to inhibit growth of E. coli [406]. Therefore, it is promising to explore applications of antibacterial metallic glasses in healthcare ranging from hospital public facilities to surgical instruments and implants.
BMGs frequently includes heavy metal elements to tune various material properties, for instance, Cu and Ag is commonly used in Zr-based BMGs to attain good glass formation ability [407,408]. However, the presence of these biocidal elements implies that there may be an antimicrobial potential of BMGs. In Huang's study [191], although bacteria killing function of Zr-based BMGs on S. aureus was observed in the direct contact test, while the alloys lacked antibacterial effect in the more aggressive dynamic immersion experimental settings, which possibly suggested the killing efficacy was directly related to the release of Cu ions.
Thin film metallic glasses (TFMGs) have recently emerged as alternative advanced surfaces for many applications [409]. It was reported the strong antibacterial ability of TFMG was ascribed to their amorphous rough surface, hydrophobic properties and released antibacterial element ions [192]. In particular, Zr-based TFMGs exhibit promising antibacterial properties [192,[410], [411], [412], [413], [414], [415], [416], [417]].
13. Antibacterial mechanisms
Antibacterial metals and alloys are not a new concept. However, antibacterial mechanism of antibacterial metals and alloys is still unclear, including the dissolution of metal ions such as Ag, Cu and Zn, the rising of pH value, the direct contact killing mechanism, the electron transfer, and the surface structure, etc.
13.1. Antibacterial mechanism of metal ions
Antibacterial metal ions such as silver (Ag+), zinc (Zn2+), and copper (Cu2+) are well known for their strong broad-spectrum antibacterial activity [93,113]. It has been widely accepted that metal ions, such as Ag+, Cu2+, Zn2+, kill bacteria by inducing the production of reactive oxygen species (ROS) [113]. ROS is the reduction products of oxygen, such as peroxides, superoxides, hydroxyl radicals and singlet oxygen, which destroy the cell membrane of bacteria, as illustrated in Fig. 24.
Fig. 24.
Antibacterial mechanism of metal ion [115], copyright 2016, Elsevier Inc.
Antibacterial activity of the materials that release metal ions (Cu2+, Zn2+, Ag+ … etc.) involves main distinct parts, as following: firstly, metal ions on the surface form strong bonds with amino and carboxyl groups of bacterium membrane and proteins, and disable them and lead to structural changes. Secondly, a bacterial membrane with structural changes presents an important permeability increase, making the bacterium unable to monitor properly the transport through the plasma membrane. In the final stage, metal ions interact with nucleic acids from microorganisms preventing replication and causing cell death.
The minimum inhibitory concentrations (MICs) of silver ions against S. aureus and E. coli were both in a range of 25.4–2040 μg/L [113]. Ning et al. [113] suggested that the optimal concentration of zinc ions was 65 μg/L. To kill the bacteria, the minimum bactericidal concentrations (MBCs) of Zn2+ was 650 μg/L. Researches on Cu- or Ag- containing metal alloys, including stainless steels, titanium alloys and cobalt alloys, have indicated that the dissolved copper ions or silver ions played an important role for the antibacterial effect, which inhibits bacterial adhesion or the formation of biofilm, or leads to the collapse of some lipopolysaccharide (LPS) patches of the cell surface, and consequently alters the permeability and functionality of the outer cell membrane [418]. It was also pointed out that the more the Cu addition, the more the release of Cu ions from alloy [380]. The antibacterial rates increased with increasing Cu2+ solution concentrations. The minimum inhibitory concentrations (MICs) of Cu2+ ions were in a range of 64–7104 μg/L [113]. Nan's results [206] strongly demonstrated that DNA of E. coli was not obviously damaged when E. coli was in contact with Cu-containing stainless steel, but cell walls was seriously changed and lots of contents in the cells were leaked, which means that the steel killed the bacterium by damaging the cell membrance rather than damaging the DNA of inner cells.
13.2. The direct contact sterilization mechanism
According to the antibacterial mechanism of metal ions, high metal ions should be released from alloy in order to obtain high antibacterial ability. However, the metal ion release from antibacterial alloy is very low. For example, the release amount of Cu ions from antibacterial Ti6Al4V–5Cu alloy after 20 days immersion in 0.9% NaCl solution was 2.498 ± 0.755 μg/L [272] and the Ag ion release from antibacterial Ti–3Ag (S) after 24 h immersion in 0.9% NaCl solution was only 1.9 μg/L [105], much less than MIC for Cu2+ of 64–7104 μg/L and for Ag+ of 25.4–2040 μg/L [113,419,420]. Table 19 summaries Cu or Ag ions release and reported antibacterial properties. Actually, previous studies have demonstrated that metallic copper [421], 316L-Cu-La-Ce stainless steel [148], La-containing antibacterial stainless steel [147] and Ti–Cu sintered alloy [18] could only kill the bacteria on the surface and had no antibacterial ability to the surrounding bacteria.
Table 19.
Cu or Ag ions release concentration Cu or Ag ions release concentration and related antibacterial properties from reported antibacterial alloy.
| Ion release concentration | The immersion solution | Antibacterial (%) | Bacteria | ref |
|---|---|---|---|---|
| 22.5 ppb Cu | 0.9% NaCl | 98.3 | S. aureus | [216] |
| 11.5 ± 4.7 ppb Cu | artificial seawater | 33.1 | P. aeruginosa | [224] |
| 25.7 ± 7.1 ppb Cu | 56.0 | |||
| 37.8 ± 8.9 ppb Cu | 70.3 | |||
| 1000 ppb Cu | supernatant of media cultured | >90 | E. coli; S. aureus | [214] |
| 2.0 ppb Cu | tap water | 40 | microorganism | [208] |
| 7.1 ppb Cu | 95.9 | |||
| 8.1 ppb Cu | 75 | |||
| about 4 ppb Ag | 0.9% NaCl | <21 | S. aureus | [74] |
| 0.63 ppb Ag | 0.9% NaCl | 38.0 | S. aureus | [71] |
| 29.657 ± 3.926 ppb Ag | antibacterial test solution | |||
| 0.78 ppb Ag | 0.9% NaCl | 57.0 | ||
| 36.519 ± 3.340 ppb Ag | antibacterial test solution | |||
| 1.06 ppb Ag | 0.9% NaCl | 67 | ||
| 49.961 ± 8.081 ppb Ag | antibacterial test solution | |||
| 1 day: < 5 ppb, 7 day: 105 ppb | 0.9% NaCl | 99.9 | S. aureus | [105] |
Note: The default antibacterial method is the plate counting method for one day.
Meanwhile, more research results have shown that the antibacterial ability of titanium alloy is closely related to the precipitation of Ag- or Cu-containing compounds. Zhang et al. [252] believed that the Ti2Cu phase played a key role in the antibacterial mechanism according to the research of Ti–Cu alloys. Peng et al. [272] discovered that the antibacterial performance of larger globule Ti2Cu and bulk β phases was better than the small ones. Chen et al. [268] suggested that the size and amount of Ag-containing compounds had a significant influence on the antibacterial properties and concluded that the antibacterial mechanism of different Ti–Ag alloys was a mixture of the direct contact antibacterial mechanism by Ti2Ag phases and Ag ions antibacterial mechanism. More recently, Shi et al. [71] confirmed that nearly 90% antibacterial ability of Ti–Ag alloys came from the precipitation of Ti2Ag in a contact sterilization mode and 10% antibacterial ability came from Ag ion sterilization.
In summary, Cu- or Ag-containing particles in nano-scale or microscale size, resists the adhesion of bacterium [193,272], the formation of biofilm [255] and kill the bacterial on the surface, as an example shown in Fig. 25. However, the exact antibacterial mechanism in the contact sterilization mode is still unclear.
Fig. 25.
The direct contact killing mechanism [268], copyright 2017, Elsevier B.V. a) nano-scale and b) micro-scale Ag- or Cu containing particles on the surface resist the bacteria adhesion, the formation of biofilm and kill the adhered bacteria.
13.3. Rising of pH value
Biodegradable magnesium and magnesium alloys with Ag or Cu elements also show strong antibacterial activity. The major soluble corrosion products of magnesium and magnesium alloys in aqueous physiological solutions are magnesium ions and hydroxide ions, thereby, the increase in solution pH and the increase in Mg ion concentrations caused by fast degradation of magnesium alloy and the formation of Mg(OH)2 on the surface might possibly contribute to the antibacterial property of magnesium and magnesium alloys [347]. As an example, Table 20 lists the relationship between antibacterial rate and Mg ion concentration reported in literatures, which indicates the addition of Mg2+ alone would not inhibit bacterial growth [345,352].
Table 20.
The relationship between antibacterial ability and Mg ion concentration.
| Solution | Mg ion concentration (mM) | Bacterium | Antibacterial ability | Ref |
|---|---|---|---|---|
| MH broth + MgCl2 salt | 0.16 | E. coli; S. aureus; P. aeruginosa | No | [345] |
| MH broth + NaCl salt | 5.95 | No | ||
| LB medium + magnesium granules + adjust to the pH of 7 | 5.5 ± 1.1 | P. aeruginosa | No | [352] |
| 7.7 ± 0.7 | ||||
| 9.7 ± 0.9 |
Note: a) MH broth: Mueller Hinton Broth, b) LB medium: Luria-Bertani medium.
On the contrary, more researches suggested there existed a relationship between pH value and the antibacterial ability, as summarized in Table 21. These results demonstrate that bacteria can be alive usually in an environment with pH range of 6.0–8.0 while both acid and alkali environments are not suitable for the survival of bacteria. When the corrosion supernatants were adjusted to a neutral pH, the inhibition effect of the bacterial was completely abolished [352,358]. In Qin's study [355], the antibacterial effect of Mg increased with the extended degradation time, which indicated the antibacterial activity was proportional to the pH value. In suitable pH environment, bacteria can maintain a cytoplasmic pH that is compatible with the optimal functional and structural integrity of the cytoplasmic proteins [422,423]. Due to the fast degradation rate of magnesium and magnesium alloys, pH value can reach at 11.0 or even higher, thus the rising in pH value should be response to the high antibacterial ability of magnesium and magnesium alloys. Although it is believed that the alkalinity contributed to bacteria death, there may be other factors affect antibacterial properties. For example, magnesium hydroxide and magnesium oxide particles formed during magnesium degradation may contribute to bacteria death [424,425].
Table 21.
The relationship between antibacterial ability and pH value.
| Solution | pH value | bacterium | Antibacterial ability | Ref |
|---|---|---|---|---|
| MH broth + NaOH | 8 |
E. coli; P.aeruginosa; S. aureus |
No | [345] |
| 9 | Yes | |||
| 10 | Yes | |||
| Pure Mg + bacterial suspensions | 8.5 |
E. coli; S. aureus |
Yes (>99.9%) | [346] |
| 10 | ||||
| Pure AZ31 + bacterial suspensions | 8.2 | |||
| 9.5 | ||||
| LB culture medium + NaOH | 7 | P. aeruginosa | No | [352] |
| 7.5 | ||||
| 8 | ||||
| 8.5 | ||||
| 9 | Yes (restrict bacterial proliferation) | |||
| 9.5 | Yes (further restrict) | |||
| Pure Mg + broth | 9.1 |
E. coli; S. epidermidis; S. aureus |
70.5%, 63.8%, 65.2%, respectively | [355] |
| 10.07 | 88.8%, 83%, 81.4%, respectively | |||
| pure Mg + PBS | 7.4 (0–120 h) | S. aureus | No | [358] |
| pure Mg + PBS | 12 | 100% |
Note: a) MH broth: Mueller Hinton broth, b) LB culture medium: Luria-Bertani culture medium.
13.4. The electron transfer
Besides above mentioned antibacterial mechanisms, electron transfer in bacterial activities is another widely accepted antibacterial mechanism [30,[426], [427], [428], [429], [430], [431]]. In fact, electron transfer is a crucial step in many bacterial activities. For instance, by means of electron transfer, bacteria complete respiration on the cell membrane to supply energy for cell growth, proliferation and maintenance [[432], [433], [434]], but on the other hand, disturbing electron transfer in bacteria can raise the production of reactive oxygen species (ROS) to hinder growth [435], as schematically shown in Fig. 26.
Fig. 26.
Schematic diagram of antibacterial process by electron transfer [436], copyright 2019, American Chemical Society.
Many studies have shown that electron transfer plays an important role in destroying electron transport in bacteria. Wang et al. [431] discovered that the antibacterial effects of Ag-NPs@Ti relied on a conductive substrate. Electrons transfer took place between the Ag-NPs and Ti substrate and produced ROS in the solution to elevate the oxidative stress at the interface. In the research of capacitive carbon-doped TiO2 nanotubes, Wang et al. [437] confirmed that electron transfer observed during early contact contributed to the surface-dependent antibacterial process. In addition, a lot of researches have realized the sterilization effect by constructing charged material surface. Van [438] have reported that a positively charged carbon surface can reduce the viability of bacteria. Positive or negative surface charges have also been found to promote the antibacterial efficiency of chitosan and inhibit adherence of Gram-negative bacteria on polymeric materials [439,440]. Further mechanistic studies indicated that surface charges can disrupt the membrane potential of bacterial cells producing irreversible damage in the membrane structure [441,442].
The electron transfer between metal ions in aqueous solution and catalytic enzyme affects the performance of bio-electronic systems (BESs). Some metal ions produced ROS by Fenton reactions, and other metal ion bond protein sulfhydryl group on bacterial membrane, which can maintain the integrity of bacterial DNA and cause the destruction of bacterial membrane, so as to achieve the effect of antibacterial effect [443,444].
14. Future
Strong and long-term antibacterial ability of antibacterial metals and alloys as well as good corrosion resistance and mechanical properties all demonstrate the potential application of antibacterial metal alloy in the future. However, there are still some concerns before the clinical application:
-
(1)
The antibacterial mechanism. Although several antibacterial mechanisms have been discussed in detail so far, the antibacterial mechanism is still not clear. Due to this reason, the design and preparation of antibacterial metal alloy still depends mainly on element alloying by the addition of proper Cu or Ag element.
-
(2)
Potential toxicity of Ag ion or Cu ion release. Lots of in vitro investigation have demonstrated that metal ion release from antibacterial alloy is very low and the antibacterial alloys exhibit good cell compatibility. In vivo results also confirm the biocompatibility of antibacterial metal alloys. However, the metal ion release, especially Ag ion and Cu ion, might cause long-term cytotoxicity or side-effect to human body.
-
(3)
Surface bioactivity. Antibacterial stainless steel, antibacterial titanium alloys and antibacterial cobalt alloys are still inert to cells and tissues. Surface biomodification to improve or enhance the cell response is still necessary, which might cause reduction in antibacterial activity on the other hand. Therefore, selection of surface biomodification and its influence on the cell response and antibacterial activity should be investigated in detail.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to acknowledge the financial support from National Natural Science Foundation of China (no. 31971253/C1002). Authors are also thankful to Xuefeng Song, Shuo Liu, Jiayu Wang, Shuang Cao and Ming Lu for their help in the preparation of this manuscript. Special thanks for the instrumental analysis of the Analytical and Testing Center, Northeastern University.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2021.01.030.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Reichman D.E., Greenberg J.A. Reducing surgical site infections. A review. Rev. Obstet. Gynecol. 2009;2:212–221. [PMC free article] [PubMed] [Google Scholar]
- 2.Grischke J., Eberhard J., Stiesch M. Antimicrobial dental implant functionalization strategies —a systematic review. Dent. Mater. J. 2016;35:545–558. doi: 10.4012/dmj.2015-314. [DOI] [PubMed] [Google Scholar]
- 3.Duske K., Jablonowski L., Koban I., Matthes R., Holtfreter B., Sckell A. Cold atmospheric plasma in combination with mechanical treatment improves osteoblast growth on biofilm covered titanium discs. Biomaterials. 2015;52:327–334. doi: 10.1016/j.biomaterials.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 4.Norowski P.A., Bumgardner J.D. Biomaterial and antibiotic strategies for peri-implantitis: a review. J. Biomed. Mater. Res. B Appl. Biomater. 2009;88B:530–543. doi: 10.1002/jbm.b.31152. [DOI] [PubMed] [Google Scholar]
- 5.Pye A.D., Lockhart D.E.A., Dawson M.P., Murray C.A., Smith A.J. A review of dental implants and infection. J. Hosp. Infect. 2009;72:104–110. doi: 10.1016/j.jhin.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Li M., Ma Z., Zhu Y., Xia H., Yao M.Y., Chu X. Toward a molecular understanding of the antibacterial mechanism of copper-bearing titanium alloys against Staphylococcus aureus. Adv. Healthcare Mater. 2016;5:557–566. doi: 10.1002/adhm.201500712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helfet D.L., Haas N.P., Schatzker J., Matter P., Moser R., Hanson B. AO philosophy and principles of fracture management - its evolution and evaluation. J. Bone Joint Surg. Am. 2003;85A:1156–1160. [PubMed] [Google Scholar]
- 8.Laffer R.R., Graber P., Ochsner P.E., Zimmerli W. Outcome of prosthetic knee-associated infection: evaluation of 40 consecutive episodes at a single centre. Clin. Microbiol. Infect. 2006;12:433–439. doi: 10.1111/j.1469-0691.2006.01378.x. [DOI] [PubMed] [Google Scholar]
- 9.Wu C.L., Qu X.H., Liu F.X., Li H.W., Mao Y.Q., Zhu Z.N. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PloS One. 2014;9 doi: 10.1371/journal.pone.0095300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessler B., Sendi P., Graber P., Knupp M., Zwicky L., Hintermann B. Risk factors for periprosthetic ankle joint infection: a case-control study. J. Bone Joint Surg. Am. 2012;94A:1871–1876. doi: 10.2106/JBJS.K.00593. [DOI] [PubMed] [Google Scholar]
- 11.Liao Y.L., Yao Y.C., Yu Y.L., Zeng Y. Enhanced antibacterial activity of curcumin by combination with metal ions. Colloid Interface Sci. Commun. 2018;25:1–6. [Google Scholar]
- 12.Campoccia D., Montanaro L., Arciola C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials. 2013;34:8533–8554. doi: 10.1016/j.biomaterials.2013.07.089. [DOI] [PubMed] [Google Scholar]
- 13.Zhao L.Z., Chu P.K., Zhang Y.M., Wu Z.F. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009;91B:470–480. doi: 10.1002/jbm.b.31463. [DOI] [PubMed] [Google Scholar]
- 14.Sahrmann P., Ronay V., Hofer D., Attin T., Jung R.E., Schmidlin P.R. In vitro cleaning potential of three different implant debridement methods. Clin. Oral Implants Res. 2015;26:314–319. doi: 10.1111/clr.12322. [DOI] [PubMed] [Google Scholar]
- 15.John G., Becker J., Schwarz F. Rotating titanium brush for plaque removal from rough titanium surfaces - an in vitro study. Clin. Oral Implants Res. 2014;25:838. doi: 10.1111/clr.12147. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D., Ren L., Zhang Y., Xue N., Yang K., Zhong M. Antibacterial activity against Porphyromonas gingivalis and biological characteristics of antibacterial stainless steel. Colloids Surf. B Biointerfaces. 2013;105:51–57. doi: 10.1016/j.colsurfb.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Shirai T., Tsuchiya H., Shimizu T., Ohtani K., Zen Y., Tomita K. Prevention of pin tract infection with titanium-copper alloys. J. Biomed. Mater. Res. B Appl. Biomater. 2009;91B:373–380. doi: 10.1002/jbm.b.31412. [DOI] [PubMed] [Google Scholar]
- 18.Zhang E.L., Li F.B., Wang H.Y., Liu J., Wang C.M., Li M.Q. A new antibacterial titanium-copper sintered alloy: preparation and antibacterial property. Mater. Sci. Eng. C. 2013;33:4280–4287. doi: 10.1016/j.msec.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Feng Y.S., Zhu S.J., Wang L.G., Chang L., Hou Y.C., Guan S.K. Fabrication and characterization of biodegradable Mg-Zn-Y-Nd-Ag alloy: microstructure, mechanical properties, corrosion behavior and antibacterial activities. Bioact. Mater. 2018;3:225–235. doi: 10.1016/j.bioactmat.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang E.L., Liu C. A new antibacterial Co-Cr-Mo-Cu alloy: preparation, biocorrosion, mechanical and antibacterial property. Mater. Sci. Eng. C. 2016;69:134–143. doi: 10.1016/j.msec.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y.J., Ren L., Wu S.Q., Yang C.G., Lin W.L., Xiao S.L. CoCrWCu alloy with antibacterial activity fabricated by selective laser melting: densification, mechanical properties and microstructural analysis. Powder Technol. 2018;325:289–300. [Google Scholar]
- 22.Ferraris S., Spriano S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C. 2016;61:965–978. doi: 10.1016/j.msec.2015.12.062. [DOI] [PubMed] [Google Scholar]
- 23.Mitik-Dineva N., Wang J., Truong V.K., Stoddart P., Malherbe F., Crawford R.J. Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus attachment patterns on glass surfaces with nanoscale roughness. Curr. Microbiol. 2009;58:268–273. doi: 10.1007/s00284-008-9320-8. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y.P., Zhao S.Y., Li G.Q., Zhang S.F., Zhao R.F., Dong A. Preparation and in vitro antibacterial properties of anodic coatings co-doped with Cu, Zn, and P on a Ti-6Al-4V alloy. Mater. Chem. Phys. 2020:241. [Google Scholar]
- 25.He X.J., Zhang G.N., Wang X., Hang R.Q., Huang X.B., Qin L. Biocompatibility, corrosion resistance and antibacterial activity of TiO2/CuO coating on titanium. Ceram. Int. 2017;43:16185–16195. [Google Scholar]
- 26.Hadidi M., Bigham A., Saebnoori E., Hassanzadeh-Tabrizi S.A., Rahmati S., Alizadeh Z.M. Electrophoretic-deposited hydroxyapatite-copper nanocomposite as an antibacterial coating for biomedical applications. Surf. Coating. Technol. 2017;321:171–179. [Google Scholar]
- 27.Cao H., Liu X., Meng F., Chu P.K. Biological actions of silver nanoparticles embedded in titanium controlled by micro-galvanic effects. Biomaterials. 2011;32:693–705. doi: 10.1016/j.biomaterials.2010.09.066. [DOI] [PubMed] [Google Scholar]
- 28.Li J.B., Qiao Y.Q., Ding Z.H., Liu X.Y. Microstructure and properties of Ag/N dual ions implanted titanium. Surf. Coating. Technol. 2011;205:5430–5436. [Google Scholar]
- 29.Zhang F., Wolf G.K., Wang X.H., Liu X.H. Surface properties of silver doped titanium oxide films. Surf. Coating. Technol. 2001;148:65–70. [Google Scholar]
- 30.Cao H.L., Qiao Y.Q., Liu X.Y., Lu T., Cui T., Meng F.H. Electron storage mediated dark antibacterial action of bound silver nanoparticles: smaller is not always better. Acta Biomater. 2013;9:5100–5110. doi: 10.1016/j.actbio.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Wojcieszak D., Mazur M., Kalisz M., Grobelny M. Influence of Cu, Au and Ag on structural and surface properties of bioactive coatings based on titanium. Mater. Sci. Eng. C. 2017;71:1115–1121. doi: 10.1016/j.msec.2016.11.091. [DOI] [PubMed] [Google Scholar]
- 32.Sun D., Xu D.K., Yang C.G., Chen J., Shahzad M.B., Sun Z.Q. Inhibition of Staphylococcus aureus biofilm by a copper-bearing 317L-Cu stainless steel and its corrosion resistance. Mater. Sci. Eng. C. 2016;69:744–750. doi: 10.1016/j.msec.2016.07.050. [DOI] [PubMed] [Google Scholar]
- 33.Zheng Y.F., Zhang B.B., Wang B.L., Wang Y.B., Li L., Yang Q.B. Introduction of antibacterial function into biomedical TiNi shape memory alloy by the addition of element Ag. Acta Biomater. 2011;7:2758–2767. doi: 10.1016/j.actbio.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Hou X.G., Mao D., Ma H.Y., Ai Y.K., Zhao X.L., Deng J.H. Antibacterial ability of Ag-TiO2 nanotubes prepared by ion implantation and anodic oxidation. Mater. Lett. 2015;161:309–312. [Google Scholar]
- 35.Hou X.G., Ma H.Y., Liu F., Deng J.H., Ai Y.K., Zhao X.L. Synthesis of Ag ion-implanted TiO2 thin films for antibacterial application and photocatalytic performance. J. Hazard Mater. 2015;299:59–66. doi: 10.1016/j.jhazmat.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Qin H., Cao H.L., Zhao Y.C., Jin G.D., Cheng M.Q., Wang J.X. Antimicrobial and osteogenic properties of silver-ion-implanted stainless steel. ACS Appl. Mater. Interfaces. 2015;7:10785–10794. doi: 10.1021/acsami.5b01310. [DOI] [PubMed] [Google Scholar]
- 37.Li J.H., Liu X.Y., Qiao Y.Q., Zhu H.Q., Ding C.X. Antimicrobial activity and cytocompatibility of Ag plasma-modified hierarchical TiO2 film on titanium surface. Colloids Surf. B Biointerfaces. 2014;113:134–145. doi: 10.1016/j.colsurfb.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X.L., Wang W.J., Zhang Y.J., Zeng T., Jia C.H., Chang L.X. Loading Cu-doped magnesium oxide onto surface of magnetic nanoparticles to prepare magnetic disinfectant with enhanced antibacterial activity. Colloids Surf. B Biointerfaces. 2018;161:433–441. doi: 10.1016/j.colsurfb.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Yao X.H., Zhang X.Y., Wu H.B., Tian L.H., Ma Y., Tang B. Microstructure and antibacterial properties of Cu-doped TiO2 coating on titanium by micro-arc oxidation. Appl. Surf. Sci. 2014;292:944–947. [Google Scholar]
- 40.Zhu W., Zhang Z.X., Gu B.B., Sun J.Y., Zhu L.X. Biological activity and antibacterial property of nano-structured TiO2 coating incorporated with Cu prepared by micro-arc oxidation. J. Mater. Sci. Technol. 2013;29:237–244. [Google Scholar]
- 41.Yu Y.Q., Jin G.D., Xue Y., Wang D.H., Liu X.Y., Sun J. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017;49:590–603. doi: 10.1016/j.actbio.2016.11.067. [DOI] [PubMed] [Google Scholar]
- 42.Jin G.D., Cao H.L., Qiao Y.Q., Meng F.H., Zhu H.Q., Liu X.Y. Osteogenic activity and antibacterial effect of zinc ion implanted titanium. Colloids Surf. B Biointerfaces. 2014;117:158–165. doi: 10.1016/j.colsurfb.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 43.Huo K.F., Zhang X.M., Wang H.R., Zhao L.Z., Liu X.Y., Chu P.K. Osteogenic activity and antibacterial effects on titanium surfaces modified with Zn-incorporated nanotube arrays. Biomaterials. 2013;34:3467–3478. doi: 10.1016/j.biomaterials.2013.01.071. [DOI] [PubMed] [Google Scholar]
- 44.Qin H., Cao H.L., Zhao Y.C., Zhu C., Cheng T., Wang Q.J. In vitro and in vivo anti-biofilm effects of silver nanoparticles immobilized on titanium. Biomaterials. 2014;35:9114–9125. doi: 10.1016/j.biomaterials.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 45.Burghardt I., Luthen F., Prinz C., Kreikemeyer B., Zietz C., Neumann H.G. A dual function of copper in designing regenerative implants. Biomaterials. 2015;44:36–44. doi: 10.1016/j.biomaterials.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 46.Roknian M., Fattah-alhosseini A., Gashti S.O., Keshavarz M.K. Study of the effect of ZnO nanoparticles addition to PEO coatings on pure titanium substrate: microstructural analysis, antibacterial effect and corrosion behavior of coatings in Ringer's physiological solution. J. Alloys Compd. 2018;740:330–345. [Google Scholar]
- 47.Mirak M., Alizadeh M., Ghaffari M., Ashtiani M.N. Characterization, mechanical properties and corrosion resistance of biocompatible Zn-HA/TiO2 nanocomposite coatings. J. Mech. Behav. Biomed. Mater. 2016;62:282–290. doi: 10.1016/j.jmbbm.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 48.Luo Q.M., Cao H.L., Wang L.Y., Ma X.H., Liu X.Y. ZnO@ZnS nanorod-array coated titanium: good to fibroblasts but bad to bacteria. J. Colloid Interface Sci. 2020;579:50–60. doi: 10.1016/j.jcis.2020.06.055. [DOI] [PubMed] [Google Scholar]
- 49.Yang T.T., Qian S., Qiao Y.Q., Liu X.Y. Cytocompatibility and antibacterial activity of titania nanotubes incorporated with gold nanoparticles. Colloids Surf. B Biointerfaces. 2016;145:597–606. doi: 10.1016/j.colsurfb.2016.05.073. [DOI] [PubMed] [Google Scholar]
- 50.Shuai C.J., Guo W., Wu P., Yang W.J., Hu S., Xia Y. A graphene oxide-Ag co-dispersing nanosystem: dual synergistic effects on antibacterial activities and mechanical properties of polymer scaffolds. Chem. Eng. J. 2018;347:322–333. [Google Scholar]
- 51.Ahmed K.B.A., Raman T., Veerappan A. Future prospects of antibacterial metal nanoparticles as enzyme inhibitor. Mater. Sci. Eng. C. 2016;68:939–947. doi: 10.1016/j.msec.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 52.Liu R., Memarzadeh K., Chang B., Zhang Y.M., Ma Z., Allaker R.P. Antibacterial effect of copper bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci. Rep. 2016;6 doi: 10.1038/srep29985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X.Y., Zhang G.N., Chai M.Z., Yao X.H., Chen W.Y., Chu P.K. Synergistic antibacterial activity of physical-chemical multi-mechanism by TiO2 nanorod arrays for safe biofilm eradication on implant. Bioact. Mater. 2021;6:12–25. doi: 10.1016/j.bioactmat.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang B., Tan L., Liu X.M., Li J., Wu S.L. A facile fabrication of novel stuff with antibacterial property and osteogenic promotion utilizing red phosphorus and near-infrared light. Bioact. Mater. 2019;4:17–21. doi: 10.1016/j.bioactmat.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Q., Liu X.M., Tan L., Cui Z.D., Li Z.Y., Liang Y.Q. An UV to NIR-driven platform based on red phosphorus/graphene oxide film for rapid microbial inactivation. Chem. Eng. J. 2020;383:123088. [Google Scholar]
- 56.Teng X.F., Liu X.M., Cui Z.D., Zheng Y.F., Chen D.F., Li Z.Y. Rapid and highly effective bacteria-killing by polydopamine/IR780@MnO2-Ti using near-infrared light. Prog. Nat. Sci.: Mater. Int. 2020;30:677–685. [Google Scholar]
- 57.Han D.L., Li Y., Liu X.M., Li B., Han Y., Zheng Y.F. Rapid bacteria trapping and killing of metal-organic frameworks strengthened photo-responsive hydrogel for rapid tissue repair of bacterial infected wounds. Chem. Eng. J. 2020:125194. [Google Scholar]
- 58.Zhu M., Liu X.M., Tan L., Cui Z.D., Liang Y.Q., Li Z.Y. Photo-responsive chitosan/Ag/MoS2 for rapid bacteria-killing. J. Hazard Mater. 2020:383. doi: 10.1016/j.jhazmat.2019.121122. [DOI] [PubMed] [Google Scholar]
- 59.Park S.W., Lee D., Choi Y.S., Jeon H.B., Lee C.-H., Moon J.-H. Mesoporous TiO2 implants for loading high dosage of antibacterial agent. Appl. Surf. Sci. 2014;303:140–146. [Google Scholar]
- 60.Zhang H.Z., Sun Y., Tian A., Xue X.X., Wang L., Alquhali A. Improved antibacterial activity and biocompatibility on vancomycin-loaded TiO2 nanotubes: in vivo and in vitro studies. Int. J. Nanomed. 2013;8:4379–4389. doi: 10.2147/IJN.S53221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tu J.J., Yu M.F., Lu Y., Cheng K., Weng W.J., Lin J. Preparation and antibiotic drug release of mineralized collagen coatings on titanium. J. Mater. Sci. Mater. Med. 2012;23:2413–2423. doi: 10.1007/s10856-012-4692-5. [DOI] [PubMed] [Google Scholar]
- 62.Liu Z.H., Zhu Y.Z., Liu X.M., Yeung K.W.K., Wu S.L. Construction of poly (vinyl alcohol)/poly (lactide-glycolide acid)/vancomycin nanoparticles on titanium for enhancing the surface self-antibacterial activity and cytocompatibility. Colloids Surf. B Biointerfaces. 2017;151:165–177. doi: 10.1016/j.colsurfb.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 63.Popat K.C., Eltgroth M., Latempa T.J., Grimes C.A., Desai T.A. Decreased Staphylococcus epidermis adhesion and increased osteoblast functionality on antibiotic-loaded titania nanotubes. Biomaterials. 2007;28:4880–4888. doi: 10.1016/j.biomaterials.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 64.Davidson H., Poon M., Saunders R., Shapiro I.M., Hickok N.J., Adams C.S. Tetracycline tethered to titanium inhibits colonization by Gram-negative bacteria. J. Biomed. Mater. Res. B Appl. Biomater. 2015;103:1381–1389. doi: 10.1002/jbm.b.33310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lv H.B., Chen Z., Yang X.P., Cen L., Zhang X., Gao P. Layer-by-layer self-assembly of minocycline-loaded chitosan/alginate multilayer on titanium substrates to inhibit biofilm formation. J. Dent. 2014;42:1464–1472. doi: 10.1016/j.jdent.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 66.He S., Zhou P., Wang L.X., Xiong X.L., Zhang Y.F., Deng Y. Antibiotic-decorated titanium with enhanced antibacterial activity through adhesive polydopamine for dental/bone implant. J. R. Soc. Interface. 2014;11 doi: 10.1098/rsif.2014.0169. 20140169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cortizo M.C., Oberti T.G., Cortizo M.S., Cortizo A.M., Fernandez Lorenzo de Mele M.A. Chlorhexidine delivery system from titanium/polybenzyl acrylate coating: evaluation of cytotoxicity and early bacterial adhesion. J. Dent. 2012;40:329–337. doi: 10.1016/j.jdent.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 68.Li C.L., Zhu J.H., Wang Y.Q., Chen Y.Y., Song L.Y., Zheng W.M. Antibacterial activity of AI-Hemocidin 2, a Novel N-Terminal peptide of hemoglobin purified from arca inflata. Mar. Drugs. 2017;15 doi: 10.3390/md15070205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wimley W.C. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem. Biol. 2010;5:905–917. doi: 10.1021/cb1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Semreen M.H., El-Gamal M.I., Abdin S., Alkhazraji H., Kamal L., Hammad S. Recent updates of marine antimicrobial peptides. Saudi Pharmaceut. J. 2018;26:396–409. doi: 10.1016/j.jsps.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi A.Q., Zhu C.S., Fu S., Wang R.X., Qin G.W., Chen D.F. What controls the antibacterial activity of Ti-Ag alloy, Ag ion or Ti2Ag particles? Mater. Sci. Eng. C. 2020;109:110548. doi: 10.1016/j.msec.2019.110548. [DOI] [PubMed] [Google Scholar]
- 72.Li Y., Liu L.N., Wan P., Zhai Z.J., Mao Z.Y., Ouyang Z.X. Biodegradable Mg-Cu alloy implants with antibacterial activity for the treatment of osteomyelitis: in vitro and in vivo evaluations. Biomaterials. 2016;106:250–263. doi: 10.1016/j.biomaterials.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 73.Hu J.L., Li H.X., Wang X.Y., Yang L., Chen M., Wang R.X. Effect of ultrasonic micro-arc oxidation on the antibacterial properties and cell biocompatibility of Ti-Cu alloy for biomedical application. Mater. Sci. Eng. C. 2020:110921. doi: 10.1016/j.msec.2020.110921. [DOI] [PubMed] [Google Scholar]
- 74.Lei Z.M., Zhang H.Z., Zhang E.L., You J.H., Ma X.X., Bai X.Z. Antibacterial activities and biocompatibilities of Ti-Ag alloys prepared by spark plasma sintering and acid etching. Mater. Sci. Eng. C. 2018;92:121–131. doi: 10.1016/j.msec.2018.06.024. [DOI] [PubMed] [Google Scholar]
- 75.Liu H., Liu R., Ullah I., Zhang S.Y., Sun Z.Q., Ren L. Rough surface of copper-bearing titanium alloy with multifunctions of osteogenic ability and antibacterial activity. J. Mater. Sci. Technol. 2020;48:130–139. [Google Scholar]
- 76.Liu R., Tang Y.L., Liu H., Zeng L.L., Ma Z., Li J. Effects of combined chemical design (Cu addition) and topographical modification (SLA) of Ti-Cu/SLA for promoting osteogenic, angiogenic and antibacterial activities. J. Mater. Sci. Technol. 2020;47:202–215. [Google Scholar]
- 77.Campoccia D., Montanaro L., Arciola C.R. A review of the clinical implications of anti-infective biomaterials and infection-resistant surfaces. Biomaterials. 2013;34:8018–8029. doi: 10.1016/j.biomaterials.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 78.Sreekumari K.R., Sato Y., Kikuchi Y. Antibacterial metals - a viable solution for bacterial attachment and microbiologically influenced corrosion. Mater. Trans. 2005;46:1636–1645. [Google Scholar]
- 79.Kawakami H., Yoshida K., Nishida Y., Kikuchi Y., Sato Y. Antibacterial properties of metallic elements for alloying evaluated with application of JIS Z 2801:2000. ISIJ Int. 2008;48:1299–1304. [Google Scholar]
- 80.Miyano Y., Koyama K., Sreekumari K.R., Sato Y., Kikuchi Y. Evaluation of antibacterial ability of some pure metals. Tetsu to Hagane-J. Iron Steel Instit. Jpn. 2007;93:57–65. [Google Scholar]
- 81.Heidenau F., Mittelmeier W., Detsch R., Haenle M., Stenzel F., Ziegler G. A novel antibacterial titania coating: metal ion toxicity and in vitro surface colonization. J. Mater. Sci. Mater. Med. 2005;16:883–888. doi: 10.1007/s10856-005-4422-3. [DOI] [PubMed] [Google Scholar]
- 82.Du W.L., Niu S.S., Xu Y.L., Xu Z.R., Fan C.L. Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr. Polym. 2009;75:385–389. [Google Scholar]
- 83.Wang D.L., Lin Z.F., Wang T., Yao Z.F., Qin M.N., Zheng S.R. Where does the toxicity of metal oxide nanoparticles come from: the nanoparticles, the ions, or a combination of both? J. Hazard Mater. 2016;308:328–334. doi: 10.1016/j.jhazmat.2016.01.066. [DOI] [PubMed] [Google Scholar]
- 84.Zhu L., Elguindi J., Rensing C., Ravishankar S. Antimicrobial activity of different copper alloy surfaces against copper resistant and sensitive Salmonella enterica. Food Microbiol. 2012;30:303–310. doi: 10.1016/j.fm.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 85.Jin G.D., Cao H.L., Qiao Y.Q., Meng F.H., Zhu H.Q., Liu X.Y. Osteogenic activity and antibacterial effect of zinc ion implanted titanium. Colloids Surf. B Biointerfaces. 2014;117:158–165. doi: 10.1016/j.colsurfb.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 86.Franklin N.M., Rogers N.J., Apte S.C., Batley G.E., Gadd G.E., Casey P.S. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ. Sci. Technol. 2007;41:8484–8490. doi: 10.1021/es071445r. [DOI] [PubMed] [Google Scholar]
- 87.Tie D., Feyerabend F., Müller W.D., Schade R., Liefeith K., Kainer K.U. Antibacterial biodegradable Mg-Ag alloys. Eur. Cell. Mater. 2013;25:284–298. doi: 10.22203/ecm.v025a20. [DOI] [PubMed] [Google Scholar]
- 88.Yamamoto A., Honma R., Sumita M. Cytotoxicity evaluation of 43 metal salts using murine fibroblasts and osteoblastic cells. J. Biomed. Mater. Res. 1998;39:331–340. doi: 10.1002/(sici)1097-4636(199802)39:2<331::aid-jbm22>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 89.Schaffer J.E., Nauman E.A., Stanciu L.A. Cold drawn bioabsorbable ferrous and ferrous composite wires: an evaluation of in vitro vascular cytocompatibility. Acta Biomater. 2013;9:8574–8584. doi: 10.1016/j.actbio.2012.07.043. [DOI] [PubMed] [Google Scholar]
- 90.Wu J., Wang L.Y., He J., Zhu C.H. In vitro cytotoxicity of Cu2+, Zn2+, Ag+ and their mixtures on primary human endometrial epithelial cells. Contraception. 2012;85:509–518. doi: 10.1016/j.contraception.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 91.Wong P.K. Mutagenicity of heavy metals. Bull. Environ. Contam. Toxicol. 1988;40:597–603. doi: 10.1007/BF01688386. [DOI] [PubMed] [Google Scholar]
- 92.White R.J. An historical overview of the use of silver in wound management. Br. J. Nurs. 2001;10:S3–S8. [Google Scholar]
- 93.Slawson R.M., Van Dyke M.I., Lee H., Trevors J.T. Germanium and silver resistance, accumulation, and toxicity in microorganisms. Plasmid. 1992;27:72–79. doi: 10.1016/0147-619x(92)90008-x. [DOI] [PubMed] [Google Scholar]
- 94.Berger T.J., Spadaro J.A., Chapin S.E., Becker R.O. Electrically generated silver ions: quantitative effects on bacterial and mammalian cells. Antimicrob. Agents Chemother. 1976;9:357–358. doi: 10.1128/aac.9.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chopra I. The increasing use of silver-based products as antimicrobial agents: a useful development or a cause for concern? J. Antimicrob. Chemother. 2007;59:587–590. doi: 10.1093/jac/dkm006. [DOI] [PubMed] [Google Scholar]
- 96.Riaz M., Zia R., Ijaz A., Hussain T., Mohsin M., Malik A. Synthesis of monophasic Ag doped hydroxyapatite and evaluation of antibacterial activity. Mater. Sci. Eng. C. 2018;90:308–313. doi: 10.1016/j.msec.2018.04.076. [DOI] [PubMed] [Google Scholar]
- 97.Ansari A., Pervez S., Javed U., Abro M.I., Nawaz M.A., Qader S.A.U. Characterization and interplay of bacteriocin and exopolysaccharide-mediated silver nanoparticles as an antibacterial agent. Int. J. Biol. Macromol. 2018;115:643–650. doi: 10.1016/j.ijbiomac.2018.04.104. [DOI] [PubMed] [Google Scholar]
- 98.Pattabi R.M., Pattabi M. Antibacterial applications of silver nanoparticles. Mater. Sci. Forum. 2013;754:131–142. [Google Scholar]
- 99.Calderon V.S., Ferreri I., Henriques M., De Hosson J.T.M., Cavaleiro A., Carvalho S. Nano-galvanic coupling for enhanced Ag+ release in ZrCN-Ag films: antibacterial application. Surf. Coating. Technol. 2016;298:1–6. [Google Scholar]
- 100.Catalano P.N., Pezzoni M., Costa C., Soler-Illia GJdAA., Bellino M.G., Desimone M.F. Optically transparent silver-loaded mesoporous thin film coating with long-lasting antibacterial activity. Microporous Mesoporous Mater. 2016;236:158–166. [Google Scholar]
- 101.Durdu S., Aktug S.L., Korkmaz K., Yalcin E., Aktas S. Fabrication, characterization and in vitro properties of silver-incorporated TiO2 coatings on titanium by thermal evaporation and micro-arc oxidation. Surf. Coating. Technol. 2018;352:600–608. [Google Scholar]
- 102.Rasoulzadehzali M., Namazi H. Facile preparation of antibacterial chitosan/graphene oxide-Ag bio-nanocomposite hydrogel beads for controlled release of doxorubicin. Int. J. Biol. Macromol. 2018;116:54–63. doi: 10.1016/j.ijbiomac.2018.04.140. [DOI] [PubMed] [Google Scholar]
- 103.Ferraris S., Miola M., Cochis A., Azzimonti B., Rimondini L., Prenesti E. In situ reduction of antibacterial silver ions to metallic silver nanoparticles on bioactive glasses functionalized with polyphenols. Appl. Surf. Sci. 2017;396:461–470. [Google Scholar]
- 104.Kang M.K., Moon S.K., Kwon J.S., Kim K.M., Kim K.N. Antibacterial effect of sand blasted, large-grit, acid-etched treated Ti-Ag alloys. Mater. Res. Bull. 2012;47:2952–2955. [Google Scholar]
- 105.Chen M., Zhang E., Zhang L. Microstructure, mechanical properties, bio-corrosion properties and antibacterial properties of Ti-Ag sintered alloys. Mater. Sci. Eng. C. 2016;62:350–360. doi: 10.1016/j.msec.2016.01.081. [DOI] [PubMed] [Google Scholar]
- 106.Hardes J., Streitburger A., Ahrens H., Nusselt T., Gebert C., Winkelmann W. The influence of elementary silver versus titanium on osteoblasts behaviour in vitro using human osteosarcoma cell lines. Sarcoma. 2007;2007:26539. doi: 10.1155/2007/26539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li P.W., Kuo T.H., Chang J.H., Yeh J.M., Chan W.H. Induction of cytotoxicity and apoptosis in mouse blastocysts by silver nanoparticles. Toxicol. Lett. 2010;197:82–87. doi: 10.1016/j.toxlet.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 108.Jing H.M., Wu X.Q., Liu Y.Q., Lu M.Q., Yang K., Yao Z.M. Antibacterial property of Ce-bearing stainless steels. J. Mater. Sci. 2007;42:5118–5122. [Google Scholar]
- 109.UEP Agency. http://www.epa.gov/pesticides/factsheets/copper-alloy-products.htm.2008
- 110.Noyce J.O., Michels H., Keevil C.W. Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. J. Hosp. Infect. 2006;63:289–297. doi: 10.1016/j.jhin.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 111.Noyce J.O., Michels H., Keevil C.W. Use of copper cast alloys to control Escherichia coli O157 cross-contamination during food processing. Appl. Environ. Microbiol. 2006;72:4239–4244. doi: 10.1128/AEM.02532-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zevenhuizen L.P.T.M., Dolfing J., Eshuis E.J., Scholtenkoerselman I.J. Inhibitory effects of copper on bacteria related to the free ion concentration. Microb. Ecol. 1979;5:139–146. doi: 10.1007/BF02010505. [DOI] [PubMed] [Google Scholar]
- 113.Ning C., Wang X., Li L., Zhu Y., Li M., Yu P. Concentration ranges of antibacterial cations for showing the highest antibacterial efficacy but the least cytotoxicity against mammalian cells: implications for a new antibacterial mechanism. Chem. Res. Toxicol. 2015;28:1815–1822. doi: 10.1021/acs.chemrestox.5b00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang X. South China university of technology; 2015. Study on Antibacterial Properties and Mechanism of Metal Ions. [Google Scholar]
- 115.Wang X.L., Liu S.X., Li M., Yu P., Chu X., Li L.H. The synergistic antibacterial activity and mechanism of multicomponent metal ions-containing aqueous solutions against Staphylococcus aureus. J. Inorg. Biochem. 2016;163:214–220. doi: 10.1016/j.jinorgbio.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 116.Kargozar S., Montazerian M., Hamzehlou S., Kim H.W., Baino F. Mesoporous bioactive glasses: promising platforms for antibacterial strategies. Acta Biomater. 2018;81:1–19. doi: 10.1016/j.actbio.2018.09.052. [DOI] [PubMed] [Google Scholar]
- 117.Fernandes J.S., Gentile P., Pires R.A., Reis R.L., Hatton P.V. Multifunctional bioactive glass and glass-ceramic biomaterials with antibacterial properties for repair and regeneration of bone tissue. Acta Biomater. 2017;59:2–11. doi: 10.1016/j.actbio.2017.06.046. [DOI] [PubMed] [Google Scholar]
- 118.Tamayo L., Azócar M., Kogan M., Riveros A., Páez M. Copper-polymer nanocomposites: an excellent and cost-effective biocide for use on antibacterial surfaces. Mater. Sci. Eng. C. 2016;69:1391–1409. doi: 10.1016/j.msec.2016.08.041. [DOI] [PubMed] [Google Scholar]
- 119.Zhang E.L., Fu S., Wang R.X., Li H.X., Liu Y., Ma Z.Q. Role of Cu element in biomedical metal alloy design. Rare Met. 2019;38:476–494. [Google Scholar]
- 120.Wang P., Yuan Y.H., Xu K., Zhong H.S., Yang Y.H., Jin S.Y. Biological applications of copper-containing materials. Bioact. Mater. 2021;6:916–927. doi: 10.1016/j.bioactmat.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scheiber I.F., Mercer J.F., Dringen R. Metabolism and functions of copper in brain. Prog. Neurobiol. 2014;116:33. doi: 10.1016/j.pneurobio.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 122.Jin S.J., Ren L., Yang K. Bio-functional Cu containing biomaterials: a new way to enhance bio-adaption of biomaterials. J. Mater. Sci. Technol. 2016;32:835–839. [Google Scholar]
- 123.Squitti R., Tecchio F., Ventriglia M. The role of copper in human diet and risk of dementia. Cur. Nutr. Rep. 2015;4:114–125. [Google Scholar]
- 124.Gargiulo N., Cusano A.M., Causa F., Caputo D., Netti P.A. Silver-containing mesoporous bioactive glass with improved antibacterial properties. J. Mater. Sci. Mater. Med. 2013;24:2129–2135. doi: 10.1007/s10856-013-4968-4. [DOI] [PubMed] [Google Scholar]
- 125.Lin H.M., Zhang J., Qu F.Y., Jiang J.J., Jiang P. In vitro hydroxyapatite-forming ability and antimicrobial properties of mesoporous bioactive glasses doped with Ti/Ag. J. Nanomater. 2013;2013:24. [Google Scholar]
- 126.Zhu Y., Li X., Yang J., Wang S., Gao H., Hanagata N. Composition-structure-property relationships of the CaO-MxOy-SiO2-P2O5 (M = Zr, Mg, Sr) mesoporous bioactive glass (MBG) scaffolds. J. Mater. Chem. 2011;21:9208–9218. [Google Scholar]
- 127.Zhang J.C., Li Y.P., Yang K.N., Hao X.H. Effects of Cu2+ and Cu+ on the proliferation, differentiation and calcification of primary mouse osteoblasts in vitro. Chin. J. Inorg. Chem. 2010;26:2251–2258. [Google Scholar]
- 128.Wu C.T., Zhou Y.H., Xu M.C., Han P.P., Chen L., Chang J. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials. 2013;34:422–433. doi: 10.1016/j.biomaterials.2012.09.066. [DOI] [PubMed] [Google Scholar]
- 129.Shi F., Liu Y.M., Zhi W., Xiao D.Q., Li H.Y., Duan K. The synergistic effect of micro/nano-structured and Cu2+-doped hydroxyapatite particles to promote osteoblast viability and antibacterial activity. Biomed. Mater. 2017;12 doi: 10.1088/1748-605X/aa6c8d. [DOI] [PubMed] [Google Scholar]
- 130.Klevay L.M. Copper in legumes may lower heart disease risk. Arch. Intern. Med. 2002;162:1780. doi: 10.1001/archinte.162.15.1780. [DOI] [PubMed] [Google Scholar]
- 131.Hu G.F. Copper stimulates proliferation of human endothelial cells under culture. J. Cell. Biochem. 2015;69:326–335. doi: 10.1002/(sici)1097-4644(19980601)69:3<326::aid-jcb10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 132.Giavaresi G., Torricelli P., Fornasari P.M., Giardino R., Barbucci R., Leone G. Blood vessel formation after soft-tissue implantation of hyaluronan-based hydrogel supplemented with copper ions. Biomaterials. 2005;26:3001–3008. doi: 10.1016/j.biomaterials.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 133.Zhang X.Y., Li J.F., Wang X., Wang Y.Y., Hang R.Q., Huang X.B. Effects of copper nanoparticles in porous TiO2 coatings on bacterial resistance and cytocompatibility of osteoblasts and endothelial cells. Mater. Sci. Eng. C. 2018;82:110–120. doi: 10.1016/j.msec.2017.08.061. [DOI] [PubMed] [Google Scholar]
- 134.Zhang Y., Wang X.Y., Ma Z.L., Bai B., Liu J., Yang L. A potential strategy for in-stent restenosis: inhibition of migration and proliferation of vascular smooth muscle cells by Cu ion. Mater. Sci. Eng. C. 2020;115:111090. doi: 10.1016/j.msec.2020.111090. [DOI] [PubMed] [Google Scholar]
- 135.Thit A., Selck H., Bjerregaard H.F. Toxicity of CuO nanoparticles and Cu ions to tight epithelial cells from Xenopus laevis (A6): effects on proliferation, cell cycle progression and cell death. Toxicol. Vitro. 2013;27:1596–1601. doi: 10.1016/j.tiv.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 136.Bondarenko O., Ivask A., Käkinen A., Kahru A. Sub-toxic effects of CuO nanoparticles on bacteria: kinetics, role of Cu ions and possible mechanisms of action. Environ. Pollut. 2012;169:81–89. doi: 10.1016/j.envpol.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 137.Sarell C.J., Wilkinson S.R., Viles J.H. Substoichiometric levels of Cu2+ ions accelerate the kinetics of fiber formation and promote cell toxicity of amyloid-beta from alzheimer disease. J. Biol. Chem. 2010;285:41533–41540. doi: 10.1074/jbc.M110.171355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fontecave M., Pierre J.L. Iron: metabolism, toxicity and therapy. Biochimie. 1993;75:767–773. doi: 10.1016/0300-9084(93)90126-d. [DOI] [PubMed] [Google Scholar]
- 139.Purnama A., Hermawan H., Couet J., Mantovani D. Assessing the biocompatibility of degradable metallic materials: state-of-the-art and focus on the potential of genetic regulation. Acta Biomater. 2010;6:1800–1807. doi: 10.1016/j.actbio.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 140.Goldhaber S.B. Trace element risk assessment: essentiality vs. toxicity. Regul. Toxicol. Pharmacol. 2003;38:232–242. doi: 10.1016/s0273-2300(02)00020-x. [DOI] [PubMed] [Google Scholar]
- 141.Peuster M., Wohlsein P., Brügmann M., Ehlerding M., Seidler K., Fink C. A novel approach to temporary stenting: degradable cardiovascular stents produced from corrodible metal - results 6-18 months after implantation into New Zealand white rabbits. Heart. 2001;86:563–569. doi: 10.1136/heart.86.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Waksman R., Pakala R., Baffour R., Seabron R., Hellinga D., Tio F.O. Short-term effects of biocorrodible iron stents in porcine coronary arteries. J. Intervent. Cardiol. 2008;21:15–20. doi: 10.1111/j.1540-8183.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- 143.Peuster M., Hesse C., Schloo T., Fink C., Beerbaum P., von Schnakenburg C. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials. 2006;27:4955–4962. doi: 10.1016/j.biomaterials.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 144.Peuster M., Wohlsein P., Brügmann M., Ehlerding M., Seidler K., Fink C. A novel approach to temporary stenting: degradable cardiovascular stents produced from corrodible metal-results 6-18 months after implantation into New Zealand white rabbits. Heart. 2001;86:563–569. doi: 10.1136/heart.86.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang Q., Cao P. Degradable porous Fe-35wt.%Mn produced via powder sintering from NH4HCO3 porogen. Mater. Chem. Phys. 2015;163:394–401. [Google Scholar]
- 146.Cochis A., Azzimonti B., Della Valle C., De Giglio E., Bloise N., Visai L. The effect of silver or gallium doped titanium against the multidrug resistant Acinetobacter baumannii. Biomaterials. 2016;80:80–95. doi: 10.1016/j.biomaterials.2015.11.042. [DOI] [PubMed] [Google Scholar]
- 147.Yuan J.P., Li W., Wang C. Effect of the La alloying addition on the antibacterial capability of 316L stainless steel. Mater. Sci. Eng. C. 2013;33:446–452. doi: 10.1016/j.msec.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 148.Liu H.W., Xu D.F., Yang K., Liu H.F., Cheng Y.F. Corrosion of antibacterial Cu-bearing 316L stainless steels in the presence of sulfate reducing bacteria. Corrosion Sci. 2018;132:46–55. [Google Scholar]
- 149.Verissimo N.C., Geilich B.M., Oliveira H.G., Caram R., Webster T.J. Reducing Staphylococcus aureus growth on Ti alloy nanostructured surfaces through the addition of Sn. J. Biomed. Mater. Res. A. 2015;103A:3757–3763. doi: 10.1002/jbm.a.35517. [DOI] [PubMed] [Google Scholar]
- 150.Kang Y., Park J., Kim D.W., Kim H., Kang Y.C. Controlling the antibacterial activity of CuSn thin films by varying the contents of Sn. Appl. Surf. Sci. 2016;389:1012–1016. [Google Scholar]
- 151.Vasanthi M., Ravichandran K., Jabena Begum N., Muruganantham G., Snega S., Panneerselvam A. Influence of Sn doping level on antibacterial activity and certain physical properties of ZnO films deposited using a simplified spray pyrolysis technique. Superlattice. Microst. 2013;55:180–190. [Google Scholar]
- 152.Fielding G.A., Roy M., Bandyopadhyay A., Bose S. Antibacterial and biological characteristics of silver containing and strontium doped plasma sprayed hydroxyapatite coatings. Acta Biomater. 2012;8:3144–3152. doi: 10.1016/j.actbio.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Leung D., Spratt D.A., Pratten J., Gulabivala K., Mordan N.J., Young A.M. Chlorhexidine-releasing methacrylate dental composite materials. Biomaterials. 2005;26:7145–7153. doi: 10.1016/j.biomaterials.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 154.Lin Y.G., Yang Z.R., Cheng J., Wang L.S. Synthesis, characterization and antibacterial property of strontium half and totally substituted hydroxyapatite nanoparticles. J. Wuhan Univ. Technol.-Materials Sci. Ed. 2008;23:475–479. [Google Scholar]
- 155.Shorr E., Carter A.C. The usefulness of strontium as an adjuvant to calcium in the remineralization of the skeleton in man. Bull. Hosp. Jt. Dis. 1952;13:59–66. [PubMed] [Google Scholar]
- 156.Guida A., Towler M.R., Wall J.G., Hill R.G., Eramo S. Preliminary work on the antibacterial effect of strontium in glass ionomer cements. J. Mater. Sci. Lett. 2003;22:1401–1403. [Google Scholar]
- 157.Looney M., O'Shea H., Gunn L., Crowley D., Boyd D. An evaluation of the processing conditions, structure, and properties (biaxial flexural strength and antibacterial efficacy) of sintered strontium-zinc-silicate glass ceramics. J. Biomater. Appl. 2013;27:937–947. doi: 10.1177/0885328211430423. [DOI] [PubMed] [Google Scholar]
- 158.Ravi N.D., Balu R., Kumar T.S.S. Strontium-substituted calcium deficient hydroxyapatite nanoparticles: synthesis, characterization, and antibacterial properties. J. Am. Ceram. Soc. 2012;95:2700–2708. [Google Scholar]
- 159.Dabsie F., Gregoire G., Sixou M., Sharrock P. Does strontium play a role in the cariostatic activity of glass ionomer? J. Dent. 2009;37:554–559. doi: 10.1016/j.jdent.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 160.Brauer D.S., Karpukhina N., Kedia G., Bhat A., Law R.V., Radecka I. Bactericidal strontium-releasing injectable bone cements based on bioactive glasses. J. R. Soc. Interface. 2013;10 doi: 10.1098/rsif.2012.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bornapour M., Celikin M., Pekguleryuz M. Thermal exposure effects on the in vitro degradation and mechanical properties of Mg-Sr and Mg-Ca-Sr biodegradable implant alloys and the role of the microstructure. Mater. Sci. Eng. C. 2015;46:16–24. doi: 10.1016/j.msec.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 162.Bornapour M., Muja N., Shum-Tim P., Cerruti M., Pekguleryuz M. Biocompatibility and biodegradability of Mg-Sr alloys: the formation of Sr-substituted hydroxyapatite. Acta Biomater. 2013;9:5319–5330. doi: 10.1016/j.actbio.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 163.Brar H.S., Wong J., Manuel M.V. Investigation of the mechanical and degradation properties of Mg-Sr and Mg-Zn-Sr alloys for use as potential biodegradable implant materials. J. Mech. Behav. Biomed. Mater. 2012;7:87–95. doi: 10.1016/j.jmbbm.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 164.Gu X.N., Xie X.H., Li N., Zheng Y.F., Qin L. In vitro and in vivo studies on a Mg-Sr binary alloy system developed as a new kind of biodegradable metal. Acta Biomater. 2012;8:2360–2374. doi: 10.1016/j.actbio.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 165.Gu X.N., Zheng Y.F., Cheng Y., Zhong S.P., Xi T.F. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials. 2009;30:484–498. doi: 10.1016/j.biomaterials.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 166.Shangguan Y.M., Sun L.N., Wan P., Tan L.L., Wang C.Y., Fan X.M. Comparison study of different coatings on degradation performance and cell response of Mg-Sr alloy. Mater. Sci. Eng. C. 2016;69:95–107. doi: 10.1016/j.msec.2016.06.073. [DOI] [PubMed] [Google Scholar]
- 167.Shangguan Y.M., Wan P., Tan L.L., Fan X.M., Qin L., Yang K. Investigation of the inner corrosion layer formed in pulse electrodeposition coating on Mg-Sr alloy and corresponding degradation behavior. J. Colloid Interface Sci. 2016;481:1–12. doi: 10.1016/j.jcis.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 168.Zhao C.Y., Pan F.S., Zhang L., Pan H.C., Song K., Tang A.T. Microstructure, mechanical properties, bio-corrosion properties and cytotoxicity of as-extruded Mg-Sr alloys. Mater. Sci. Eng. C. 2017;70:1081–1088. doi: 10.1016/j.msec.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 169.Chen K., Xie X.H., Tang H.Y., Sun H., Qin L., Zheng Y.F. In vitro and in vivo degradation behavior of Mg-2Sr-Ca and Mg-2Sr-Zn alloys. Bioact. Mater. 2020;5:275–285. doi: 10.1016/j.bioactmat.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Almoudi M.M., Hussein A.S., Abu Hassan M.I., Mohamad Zain N. A systematic review on antibacterial activity of zinc against Streptococcus mutans. Saudi Dental J. 2018;30:283–291. doi: 10.1016/j.sdentj.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lynch R.J. Zinc in the mouth, its interactions with dental enamel and possible effects on caries; a review of the literature. Int. Dent. J. 2011;61(Suppl 3):46–54. doi: 10.1111/j.1875-595X.2011.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Petrini P., Arciola C.R., Pezzali I., Bozzini S., Montanaro L., Tanzi M.C. Antibacterial activity of zinc modified titanium oxide surface. Int. J. Artif. Organs. 2006;29:434–442. doi: 10.1177/039139880602900414. [DOI] [PubMed] [Google Scholar]
- 173.Zhang X.Y., Wang H.Z., Li J.F., He X.J., Hang R.Q., Huang X.B. Corrosion behavior of Zn-incorporated antibacterial TiO2 porous coating on titanium. Ceram. Int. 2016;42:17095–17100. [Google Scholar]
- 174.Wang R.Y., He X.J., Gao Y., Zhang X.Y., Yao X.H., Tang B. Antimicrobial property, cytocompatibility and corrosion resistance of Zn-doped ZrO2/TiO2 coatings on Ti6Al4V implants. Mater. Sci. Eng. C. 2017;75:7–15. doi: 10.1016/j.msec.2017.02.036. [DOI] [PubMed] [Google Scholar]
- 175.Zhang L., Gao Q., Han Y. Zn and Ag Co-doped anti-microbial TiO2 coatings on Ti by micro-arc oxidation. J. Mater. Sci. Technol. 2016;32:919–924. [Google Scholar]
- 176.Qi K.Z., Cheng B., Yu J.G., Ho W.K. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017;727:792–820. [Google Scholar]
- 177.Djurisic A.B., Leung Y.H., Ng A.M., Xu X.Y., Lee P.K., Degger N. Toxicity of metal oxide nanoparticles: mechanisms, characterization, and avoiding experimental artefacts. Small. 2015;11:26–44. doi: 10.1002/smll.201303947. [DOI] [PubMed] [Google Scholar]
- 178.Li Y., Niu J.F., Zhang W., Zhang L.L., Shang E.X. Influence of aqueous media on the ROS-mediated toxicity of ZnO nanoparticles toward green fluorescent protein-expressing Escherichia coli under UV-365 irradiation. Langmuir. 2014;30:2852–2862. doi: 10.1021/la5000028. [DOI] [PubMed] [Google Scholar]
- 179.Hu H., Zhang W., Qiao Y., Jiang X., Liu X., Ding C. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2012;8:904–915. doi: 10.1016/j.actbio.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 180.Bellanger X., Billard P., Schneider R., Balan L., Merlin C. Stability and toxicity of ZnO quantum dots: interplay between nanoparticles and bacteria. J. Hazard Mater. 2015;283:110–116. doi: 10.1016/j.jhazmat.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 181.Kavitha T., Gopalan A.I., Lee K.P., Park S.Y. Glucose sensing, photocatalytic and antibacterial properties of graphene-ZnO nanoparticle hybrids. Carbon. 2012;50:2994–3000. [Google Scholar]
- 182.Zhang L., Ding Y., Povey M., York D. ZnO nanofluids - a potential antibacterial agent. Prog. Nat. Sci. 2008;18:939–944. [Google Scholar]
- 183.Yamaguchi M., Oishi H., Suketa Y. Zinc stimulation of bone protein synthesis in tissue culture: activation of aminoacyl-tRNA synthetase. Biochem. Pharmacol. 1988;37:4075–4080. doi: 10.1016/0006-2952(88)90098-6. [DOI] [PubMed] [Google Scholar]
- 184.Yamaguchi M., Oishi H., Suketa Y. Stimulatory effect of zinc on bone formation in tissue culture. Biochem. Pharmacol. 1987;36:4007–4012. doi: 10.1016/0006-2952(87)90471-0. [DOI] [PubMed] [Google Scholar]
- 185.Yamaguchi M., Inamoto K., Suketa Y. Effect of essential trace metals on bone metabolism in weanling rats: comparison with zinc and other metals' actions. Res. Exp. Med. 1986;186:337–342. doi: 10.1007/BF01852099. [DOI] [PubMed] [Google Scholar]
- 186.Yamaguchi M., Yamaguchi R. Action of zinc on bone metabolism in rats: increases in alkaline phosphatase activity and DNA content. Biochem. Pharmacol. 1986;35:773–777. doi: 10.1016/0006-2952(86)90245-5. [DOI] [PubMed] [Google Scholar]
- 187.Yoo M.H., Lee J.Y., Lee S.E., Koh J.Y., Yoon Y.H. Protection by pyruvate of rat retinal cells against zinc toxicity in vitro, and pressure-induced ischemia in vivo. Invest. Ophthalmol. Vis. Sci. 2004;45:1523–1530. doi: 10.1167/iovs.03-1315. [DOI] [PubMed] [Google Scholar]
- 188.Rodilla V., Miles A.T., Jenner W., Hawksworth G.M. Exposure of cultured human proximal tubular cells to cadmium, mercury, zinc and bismuth: toxicity and metallothionein induction. Chem. Biol. Interact. 1998;115:71–83. doi: 10.1016/s0009-2797(98)00059-3. [DOI] [PubMed] [Google Scholar]
- 189.Kubasek J., Vojtech D., Jablonska E., Pospisilova I., Lipov J., Ruml T. Structure, mechanical characteristics and in vitro degradation, cytotoxicity, genotoxicity and mutagenicity of novel biodegradable Zn-Mg alloys. Mater. Sci. Eng. C. 2016;58:24–35. doi: 10.1016/j.msec.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 190.Ren L., Yang K., Guo L., Chai H.W. Preliminary study of anti-infective function of a copper-bearing stainless steel. Mater. Sci. Eng. C. 2012;32:1204–1209. [Google Scholar]
- 191.Huang L., Fozo E.M., Zhang T., Liaw P.K., He W. Antimicrobial behavior of Cu-bearing Zr-based bulk metallic glasses. Mater. Sci. Eng. C. 2014;39:325–329. doi: 10.1016/j.msec.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 192.Chu J.H., Lee J., Chang C.C., Chan Y.C., Liou M.L., Lee J.W. Antimicrobial characteristics in Cu-containing Zr-based thin film metallic glass. Surf. Coating. Technol. 2014;259(Part A):87–93. [Google Scholar]
- 193.Zhang Z.M., Zheng G.T., Li H.X., Yang L., Wang X.Y., Qin G.W. Anti-bacterium influenced corrosion effect of antibacterial Ti-3Cu alloy in Staphylococcus aureus suspension for biomedical application. Mater. Sci. Eng. C. 2019;94:376–384. doi: 10.1016/j.msec.2018.09.057. [DOI] [PubMed] [Google Scholar]
- 194.Bai B., Zhang E.L., Liu J.C., Zhu J.T. The anti-bacterial activity of titanium-copper sintered alloy against Porphyromonas gingivalis in vitro. Dent. Mater. J. 2016;35:659–667. doi: 10.4012/dmj.2016-001. [DOI] [PubMed] [Google Scholar]
- 195.Wang X.Y., Dong H., Liu J., Qin G.W., Chen D.F., Zhang E.L. In vivo antibacterial property of Ti-Cu sintered alloy implant. Mater. Sci. Eng. C. 2019;100:38–47. doi: 10.1016/j.msec.2019.02.084. [DOI] [PubMed] [Google Scholar]
- 196.Bekmurzayeva A., Duncanson W.J., Azevedo H.S., Kanayeva D. Surface modification of stainless steel for biomedical applications: revisiting a century-old material. Mater. Sci. Eng. C. 2018;93:1073–1089. doi: 10.1016/j.msec.2018.08.049. [DOI] [PubMed] [Google Scholar]
- 197.Morihiro H, Katsuhisa M, Naoto O, Sadayuki N. Stainless stell excellent in antibacterial property and designing property. In: Nisshin Steel Co L, editor. Japenese Laid-Open Patent Publication. Japan1996.
- 198.Naoto O, Sadayuki N, Morihiro H. High strength martensitic stainless steel excellent in antibacterial characteristic. In: Nisshin Steel Co L, editor. Japenese Laid-Open Patent Publication. Japan1996.
- 199.Chen S.H., Lu M.Q., Zhang J.D., Dong J.S., Yang K. Microstructure and antibacterial properties of Cu-contained antibacterial stainless steel. Acta Metall. Sin. 2004;40:314–318. [Google Scholar]
- 200.Yang K., Lu M.Q. Antibacterial properties of an austenitic antibacterial stainless steel and its security for human body. J. Mater. Sci. Technol. 2007;23:333–336. [Google Scholar]
- 201.Nan L., Liu Y.Q., Yang W.C., Xu H., Li Y., Lv M.Q. Study on antibacterial properties of copper-containing antibacterial stainless steel. Acta Metall. Sin. 2007;43:1065–1070. [Google Scholar]
- 202.Nan L., Yang K. Effect of Cu addition on antibacterial property of type 200 stainless steel. Mater. Technol. 2016;31:44–47. [Google Scholar]
- 203.Hong I.T., Koo C.H. Antibacterial properties, corrosion resistance and mechanical properties of Cu-modified SUS 304 stainless steel. Mater. Sci. Eng. A. 2005;393:213–222. [Google Scholar]
- 204.Qiu W.J., Lin G., Jiang L.Z., He G. Effect of Cu on properties of antibacterial austenitic stainless steel. Iron Steel. 2009;44:81–84. [Google Scholar]
- 205.Guan B., Hong S.H., Schulz C., Stanford N. The microstructure, antimicrobial properties, and corrosion resistance of Cu-bearing strip cast steel. Adv. Eng. Mater. 2020;22 [Google Scholar]
- 206.Nan L., Yang W.C., Liu Y.Q., Xu H., Li Y., Lu M.Q. Antibacterial mechanism of copper-bearing antibacterial stainless steel against E.coli. J. Mater. Sci. Technol. 2008;24:197–201. doi: 10.1007/s10856-008-3444-z. [DOI] [PubMed] [Google Scholar]
- 207.Nan L., Yang K. Cu ions dissolution from Cu-bearing antibacterial stainless steel. J. Mater. Sci. Technol. 2010;26:941–944. [Google Scholar]
- 208.Li M.J., Nan L., Xu D.K., Ren G.G., Yang K. Antibacterial performance of a Cu-bearing stainless steel against microorganisms in tap water. J. Mater. Sci. Technol. 2015;31:243–251. [Google Scholar]
- 209.Liao K.H., Ou K.L., Cheng H.C., Lin C.T., Peng P.W. Effect of silver on antibacterial properties of stainless steel. Appl. Surf. Sci. 2010;256:3642–3646. [Google Scholar]
- 210.Sreekumari K.R., Nandakumar K., Takao K., Kikuchi Y. Silver containing stainless steel as a new outlook to abate bacterial adhesion and microbiologically influenced corrosion. ISIJ Int. 2003;43:1799–1806. [Google Scholar]
- 211.Wang Q., Ren L., Li X.P., Zhang S.Y., Sercombe T.B., Yang K. Antimicrobial Cu-bearing stainless steel scaffolds. Mater. Sci. Eng. C. 2016;68:519–522. doi: 10.1016/j.msec.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 212.Zhuang Y.F., Zhang S.Y., Yang K., Ren L., Dai K.R. Antibacterial activity of copper-bearing 316L stainless steel for the prevention of implant-related infection. J. Biomed. Mater. Res. B Appl. Biomater. 2020;108:484–495. doi: 10.1002/jbm.b.34405. [DOI] [PubMed] [Google Scholar]
- 213.Xi T., Shahzad M.B., Xu D.K., Sun Z.Q., Zhao J.L., Yang C.G. Effect of copper addition on mechanical properties, corrosion resistance and antibacterial property of 316L stainless steel. Mater. Sci. Eng. C. 2017;71:1079–1085. doi: 10.1016/j.msec.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 214.Chai H.W., Guo L., Wang X.T., Fu Y.P., Guan J.L., Tan L.L. Antibacterial effect of 317L stainless steel contained copper in prevention of implant-related infection in vitro and in vivo. J. Mater. Sci. Mater. Med. 2011;22:2525–2535. doi: 10.1007/s10856-011-4427-z. [DOI] [PubMed] [Google Scholar]
- 215.Zhao J., Cao Z.Q., Ren L., Chen S.S., Zhang B.C., Liu R. A novel ureteral stent material with antibacterial and reducing encrustation properties. Mater. Sci. Eng. C. 2016;68:221–228. doi: 10.1016/j.msec.2016.04.103. [DOI] [PubMed] [Google Scholar]
- 216.Sun D., Xu D.K., Yang C.G., Shahzad M.B., Sun Z.Q., Xia J. An investigation of the antibacterial ability and cytotoxicity of a novel cu-bearing 317L stainless steel. Sci. Rep. 2016;6 doi: 10.1038/srep29244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bahmani-Oskooee M., Hossein Nedjad S., Samadi A., Kozeschnik E. Cu-bearing, martensitic stainless steels for applications in biological environments. Mater. Des. 2017;130:442–451. [Google Scholar]
- 218.Liu Y.Q., Nan L., Chen D.M., Yang K. Study of a Cu-containing martensitic antibacterial stainless steel. Rare Met. Mater. Eng. 2008;37:1380–1383. [Google Scholar]
- 219.Wang S., Yang C., Shen M., Yang K. Effect of aging on antibacterial performance of Cu-bearing martensitic stainless steel. Mater. Technol. 2014;29:257–261. [Google Scholar]
- 220.Wang S., Yang C.G., Xu D.K., Shen M.G., Nan L., Yang K. Effect of heat treatment on antibacterial performance of 3Cr13MoCu martensitic stainless steel. Acta Metall. Sin. 2014;50:1453–1460. [Google Scholar]
- 221.Wang S., Yang K., Shen M., Yang C.G. Effect of Cu content on antibacterial activity of 17-4 PH stainless steel. Mater. Technol. 2015;30:B115–B119. [Google Scholar]
- 222.Nan L., Cheng J.L., Yang K. Antibacterial behavior of a Cu-bearing type 200 stainless steel. J. Mater. Sci. Technol. 2012;28:1067–1070. [Google Scholar]
- 223.Yang S.M., Chen Y.C., Pan Y.T., Lin D.Y. Effect of silver on microstructure and antibacterial property of 2205 duplex stainless steel. Mater. Sci. Eng. C. 2016;63:376–383. doi: 10.1016/j.msec.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 224.Lou Y.T., Lin L., Xu D.K., Zhao S.Y., Yang C.G., Liu J.L. Antibacterial ability of a novel Cu-bearing 2205 duplex stainless steel against Pseudomonas aeruginosa biofilm in artificial seawater. Int. Biodeterior. Biodegrad. 2016;110:199–205. [Google Scholar]
- 225.Liu J.L., Jia R., Zhou E.Z., Zhao Y., Dou W.W., Xu D.K. Antimicrobial Cu-bearing 2205 duplex stainless steel against MIC by nitrate reducing Pseudomonas aeruginosa biofilm. Int. Biodeterior. Biodegrad. 2018;132:132–138. [Google Scholar]
- 226.Xiang H., Liu D., Chen X., Cao H., Dong X. On the microstructure and mechanical properties of silver-bearing antibacterial CD4MCu duplex stainless steels: solid solution temperature. Mater. Express. 2019;9:1067–1075. [Google Scholar]
- 227.Lin G., Shen J.C., Wang R.M. The effect of copper on the properties of ferritic antibacterial stainless steel. Funct. Mater. 2011;42:549–551. [Google Scholar]
- 228.Xie Q. Northeastern University; 2011. Microstructure and Properties of Ferritic Stainless Steel Containing Copper. [Google Scholar]
- 229.Zhao W.F., Shu J., Bi H.Y. Effect of copper on the mechanical property and corrosion behavior of 19Cr-1.6Mo ferritic stainless steel. J. Iron Steel Res. 2012;24:45–50. [Google Scholar]
- 230.Zhang Z.X. Shanghai Jiaotong University; 2007. Microstructure and Properties of Antibacterial Stainless Steel Containing Copper (Nitrogen) [Google Scholar]
- 231.Xi T., Babar Shahzad M., Xu D.K., Zhao J.L., Yang C.G., Qi M. Copper precipitation behavior and mechanical properties of Cu-bearing 316L austenitic stainless steel: a comprehensive cross-correlation study. Mater. Sci. Eng. A. 2016;675:243–252. [Google Scholar]
- 232.Ran Q.X., Li J., Xu Y.L., Xiao X.S., Yu H.F., Jiang L.Z. Novel Cu-bearing economical 21Cr duplex stainless steels. Mater. Des. 2013;46:758–765. [Google Scholar]
- 233.Zhao R.D. Liaoning University of Engineering and Technology; 2004. Effect of Copper Content on Microstructure and Properties of Low-Carbon Martensitic Antibacterial Stainless Steel. [Google Scholar]
- 234.Luo F.F., Tang Z.H., Xiao S.F., Xiang Y.L. Study on properties of copper-containing austenitic antibacterial stainless steel. Mater. Technol. 2019;34:525–533. [Google Scholar]
- 235.Nan L., Liu Y.Q., Lu M.Q., Yang K. Study on antibacterial mechanism of copper-bearing austenitic antibacterial stainless steel by atomic force microscopy. J. Mater. Sci. Mater. Med. 2008;19:3057–3062. doi: 10.1007/s10856-008-3444-z. [DOI] [PubMed] [Google Scholar]
- 236.Ujiro T., Satoh S., Staehle R.W., Smyrl W.H. Effect of alloying Cu on the corrosion resistance of stainless steels in chloride media. Corrosion Sci. 2001;43:2185–2200. [Google Scholar]
- 237.Jiang J., Xu D., Xi T., Shahzad M.B., Khan M.S., Zhao J. Effects of aging time on intergranular and pitting corrosion behavior of Cu-bearing 304L stainless steel in comparison with 304L stainless steel. Corrosion Sci. 2016;113:46–56. [Google Scholar]
- 238.Huang Y.L., Zhao J.L., Zhang J.R., Yang C.G., Zhao Y., Yang K. Optimized antibacterial treatment for the Cu-bearing 420 stainless steel. Mater. Technol. 2018;33:699–708. [Google Scholar]
- 239.Jiang J., Xu D.K., Xi T., Shahzad M.B., Khan M.S., Zhao J. Effects of aging time on intergranular and pitting corrosion behavior of Cu-bearing 304L stainless steel in comparison with 304L stainless steel. Corrosion Sci. 2016;113:46–56. [Google Scholar]
- 240.Banas J., Mazurkiewicz A. The effect of copper on passivity and corrosion behaviour of ferritic and ferritic-austenitic stainless steels. Mater. Sci. Eng. A. 2000;277:183–191. [Google Scholar]
- 241.Ren L., Nan L., Yang K. Study of copper precipitation behavior in a Cu-bearing austenitic antibacterial stainless steel. Mater. Des. 2011;32:2374–2379. [Google Scholar]
- 242.Nan L., Xu D.K., Gu T.Y., Song X., Yang K. Microbiological influenced corrosion resistance characteristics of a 304L-Cu stainless steel against Escherichia coli. Mater. Sci. Eng. C. 2015;48:228–234. doi: 10.1016/j.msec.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 243.Xia J., Yang C.G., Xu D.K., Sun D., Nan L., Sun Z.Q. Laboratory investigation of the microbiologically influenced corrosion (MIC) resistance of a novel Cu-bearing 2205 duplex stainless steel in the presence of an aerobic marine Pseudomonas aeruginosa biofilm. Biofouling. 2015;31:481–492. doi: 10.1080/08927014.2015.1062089. [DOI] [PubMed] [Google Scholar]
- 244.Yuan J.P., Li W. Antibacterial 316L stainless steel containing silver and niobium. Rare Met. Mater. Eng. 2013;42:2004–2008. [Google Scholar]
- 245.Cao Z.Q., Zhao J., Yang K. Cu-bearing stainless steel reduces cytotoxicity and crystals adhesion after ureteral epithelial cells exposing to calcium oxalate monohydrate. Sci. Rep. 2018;8:14094. doi: 10.1038/s41598-018-32388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ren L., Wong H.M., Yan C.H., Yeung K.W., Yang K. Osteogenic ability of Cu-bearing stainless steel. J. Biomed. Mater. Res. B Appl. Biomater. 2015;103:1433–1444. doi: 10.1002/jbm.b.33318. [DOI] [PubMed] [Google Scholar]
- 247.Li J.Z., Ren L., Zhang S.Y., Ren G.G., Yang K. Cu-bearing steel reduce inflammation after stent implantation. J. Mater. Sci. Mater. Med. 2015;26:114. doi: 10.1007/s10856-015-5454-y. [DOI] [PubMed] [Google Scholar]
- 248.Zhao J., Ren L., Liu M.X., Xi T., Zhang B.C., Yang K. Anti-fibrotic function of Cu-bearing stainless steel for reducing recurrence of urethral stricture after stent implantation. J. Biomed. Mater. Res. B. 2018;106:2019–2028. doi: 10.1002/jbm.b.34005. [DOI] [PubMed] [Google Scholar]
- 249.Zhao J., Ren L., Zhang B.C., Cao Z.Q., Yang K. In vitro study on infectious ureteral encrustation resistance of Cu-bearing stainless steel. J. Mater. Sci. Technol. 2017;33:1604–1609. [Google Scholar]
- 250.Wang L., Li G.Y., Ren L., Kong X.D., Wang Y.G., Han X.G. Nano-copper-bearing stainless steel promotes fracture healing by accelerating the callus evolution process. Int. J. Nanomed. 2017;12:8443–8457. doi: 10.2147/IJN.S146866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bai B., Zhang E.L., Dong H., Liu J. Biocompatibility of antibacterial Ti-Cu sintered alloy: in vivo bone response. J. Mater. Sci. Mater. Med. 2015;26:265. doi: 10.1007/s10856-015-5600-6. [DOI] [PubMed] [Google Scholar]
- 252.Zhang E.L., Wang X.Y., Chen M., Hou B. Effect of the existing form of Cu element on the mechanical properties, bio-corrosion and antibacterial properties of Ti-Cu alloys for biomedical application. Mater. Sci. Eng. C. 2016;69:1210–1221. doi: 10.1016/j.msec.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 253.Guo S., Lu Y.J., Wu S.Q., Liu L.L., He M.J., Zhao C.Q. Preliminary study on the corrosion resistance, antibacterial activity and cytotoxicity of selective-laser-melted Ti6Al4V-xCu alloys. Mater. Sci. Eng. C. 2017;72:631–640. doi: 10.1016/j.msec.2016.11.126. [DOI] [PubMed] [Google Scholar]
- 254.Krakhmalev P., Yadroitsev I., Yadroitsava I., de Smidt O. Functionalization of biomedical Ti6Al4V via in situ alloying by Cu during laser powder bed fusion manufacturing. Materials (Basel) 2017;10 doi: 10.3390/ma10101154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Liu R., Tang Y.L., Zeng L.L., Zhao Y., Ma Z., Sun Z.Q. In vitro and in vivo studies of anti-bacterial copper-bearing titanium alloy for dental application. Dent. Mater. : Off. Publ. Acad. Dental Mater. 2018;34:1112–1126. doi: 10.1016/j.dental.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 256.Ke Z.Y., Yi C.B., Zhang L., He Z.Y., Tan J., Jiang Y.H. Characterization of a new Ti-13Nb-13Zr-10Cu alloy with enhanced antibacterial activity for biomedical applications. Mater. Lett. 2019;253:335–338. [Google Scholar]
- 257.Zhang D.Y., Qiu D., Gibson M.A., Zheng Y.F., Fraser H.L., StJohn D.H. Additive manufacturing of ultrafine-grained high-strength titanium alloys. Nature. 2019;576:91–95. doi: 10.1038/s41586-019-1783-1. [DOI] [PubMed] [Google Scholar]
- 258.Tao S.C., Xu J.L., Yuan L., Luo J.M., Zheng Y.F. Microstructure, mechanical properties and antibacterial properties of the microwave sintered porous Ti-3Cu alloys. J. Alloys Compd. 2020;812:152142. [Google Scholar]
- 259.Liu X.W., Tian A., You J.H., Zhang H.Z., Wu L., Bai X.Z. Antibacterial abilities and biocompatibilities of Ti-Ag alloys with nanotubular coatings. Int. J. Nanomed. 2016;11:5743–5755. doi: 10.2147/IJN.S113674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wang X.G., Qiao J.W., Yuan F.X., Hang R.Q., Huang X.B., Tang B. In situ growth of self-organized Cu-containing nano-tubes and nano-pores on Ti90-xCu10Alx (x=0, 45) alloys by one-pot anodization and evaluation of their antimicrobial activity and cytotoxicity. Surf. Coating. Technol. 2014;240:167–178. [Google Scholar]
- 261.Liu J., Zhang X.X., Wang H.Y., Li F.B., Li M.Q., Yang K. The antibacterial properties and biocompatibility of a Ti-Cu sintered alloy for biomedical application. Biomed. Mater. 2014;9 doi: 10.1088/1748-6041/9/2/025013. [DOI] [PubMed] [Google Scholar]
- 262.Liu J., Li F.B., Liu C., Wang H.Y., Ren B.R., Yang K. Effect of Cu content on the antibacterial activity of titanium-copper sintered alloys. Mater. Sci. Eng. C. 2014;35:392–400. doi: 10.1016/j.msec.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 263.Ma Z., Li M., Liu R., Ren L., Zhang Y., Pan H. In vitro study on an antibacterial Ti-5Cu alloy for medical application. J. Mater. Sci. Mater. Med. 2016;27:91. doi: 10.1007/s10856-016-5698-1. 2016;27:91. [DOI] [PubMed] [Google Scholar]
- 264.Bao M.M., Liu Y., Wang X.Y., Yang L., Li S.Y., Ren J. Optimization of mechanical properties, biocorrosion properties and antibacterial properties of wrought Ti-3Cu alloy by heat treatment. Bioact. Mater. 2018;3:28–38. doi: 10.1016/j.bioactmat.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Moniri Javadhesari S., Alipour S., Akbarpour M.R. Biocompatibility, osseointegration, antibacterial and mechanical properties of nanocrystalline Ti-Cu alloy as a new orthopedic material. Colloids Surf. B Biointerfaces. 2020;189:110889. doi: 10.1016/j.colsurfb.2020.110889. [DOI] [PubMed] [Google Scholar]
- 266.Wu J.H., Chen K.K., Chao C.Y., Chang Y.H., Du J.K. Effect of Ti2Cu precipitation on antibacterial property of Ti-5Cu alloy. Mater. Sci. Eng. C. 2020;108:110433. doi: 10.1016/j.msec.2019.110433. [DOI] [PubMed] [Google Scholar]
- 267.Fowler L., Janson O., Engqvist H., Norgren S., Ohman-Magi C. Antibacterial investigation of titanium-copper alloys using luminescent Staphylococcus epidermidis in a direct contact test. Mater. Sci. Eng. C. 2019;97:707–714. doi: 10.1016/j.msec.2018.12.050. [DOI] [PubMed] [Google Scholar]
- 268.Chen M., Yang L., Zhang L., Han Y., Lu Z., Qin G.W. Effect of nano/micro-Ag compound particles on the bio-corrosion, antibacterial properties and cell biocompatibility of Ti-Ag alloys. Mater. Sci. Eng. C. 2017;75:906–917. doi: 10.1016/j.msec.2017.02.142. [DOI] [PubMed] [Google Scholar]
- 269.Nakajo K., Takahashi M., Kikuchi M., Takada Y., Okuno O., Sasaki K. Inhibitory effect of Ti-Ag alloy on artificial biofilm formation. Dent. Mater. J. 2014;33:389–393. doi: 10.4012/dmj.2013-334. [DOI] [PubMed] [Google Scholar]
- 270.Ma Z., Ren L., Liu R., Yang K., Zhang Y., Liao Z.H. Effect of heat treatment on Cu distribution, antibacterial performance and cytotoxicity of Ti-6Al-4V-5Cu alloy. J. Mater. Sci. Technol. 2015;31:723–732. [Google Scholar]
- 271.Ma Z., Yao M., Liu R., Yang K., Ren L., Zhang Y. Study on antibacterial activity and cytocompatibility of Ti-6Al-4V-5Cu alloy. Mater. Technol. 2015;30:B80–B85. [Google Scholar]
- 272.Peng C., Zhang S.Y., Sun Z.Q., Ren L., Yang K. Effect of annealing temperature on mechanical and antibacterial properties of Cu-bearing titanium alloy and its preliminary study of antibacterial mechanism. Mater. Sci. Eng. C. 2018;93:495–504. doi: 10.1016/j.msec.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 273.Ren L., Ma Z., Li M., Zhang Y., Liu W.Q., Liao Z.H. Antibacterial properties of Ti-6Al-4V-xCu alloys. J. Mater. Sci. Technol. 2014;30:699–705. [Google Scholar]
- 274.Macpherson A., Li X.P., McCormick P., Ren L., Yang K., Sercombe T.B. Antibacterial titanium produced using selective laser melting. JOM (J. Occup. Med.) 2017;69:2719–2724. [Google Scholar]
- 275.Ou K.L., Weng C.C., Lin Y.H., Huang M.S. A promising of alloying modified beta-type Titanium-Niobium implant for biomedical applications: microstructural characteristics, in vitro biocompatibility and antibacterial performance. J. Alloys Compd. 2017;697:231–238. [Google Scholar]
- 276.Du J.K., Chao C.Y., Chiu K.Y., Chang Y.H., Chen K.K., Wu J.H. Antibacterial properties and corrosion resistance of the newly developed biomaterial, Ti-12Nb-1Ag Alloy. Metals. 2017;7 [Google Scholar]
- 277.Takahashi M., Kikuchi M., Takada Y. Mechanical properties and microstructures of dental cast Ti-Ag and Ti-Cu alloys. Dent. Mater. J. 2002;21:270–280. doi: 10.4012/dmj.21.270. [DOI] [PubMed] [Google Scholar]
- 278.Kikuchi M., Takada Y., Kiyosue S., Yoda M., Woldu M., Cai Z. Mechanical properties and microstructures of cast Ti-Cu alloys. Dent. Mater. 2003;19:174–181. doi: 10.1016/s0109-5641(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 279.Peng C., Liu Y., Liu H., Zhang S.Y., Bai C.G., Wan Y.Z. Optimization of annealing treatment and comprehensive properties of Cu-containing Ti6Al4V-xCu alloys. J. Mater. Sci. Technol. 2019;35:2121–2131. [Google Scholar]
- 280.Kikuchi M., Takada Y., Kiyosue S., Yoda M., Woldu M., Cai Z. Mechanical properties and microstructures of cast Ti-Cu alloys. Dent. Mater. 2003;19:174–181. doi: 10.1016/s0109-5641(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 281.Alshammari Y., Yang F., Bolzoni L. Low-cost powder metallurgy Ti-Cu alloys as a potential antibacterial material. J. Mech. Behav. Biomed. 2019;95:232–239. doi: 10.1016/j.jmbbm.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 282.Wang J.W., Zhang S.Y., Sun Z.Q., Wang H., Ren L., Yang K. Optimization of mechanical property, antibacterial property and corrosion resistance of Ti-Cu alloy for dental implant. J. Mater. Sci. Technol. 2019;35:2336–2344. [Google Scholar]
- 283.Mukhopadhyay P., Singh V., Bhattacharjee A., Gogia A.K. Effect of nano Ti2Cu precipitates in Ti6Al4V2.5Cu alloy. Mater. Today: Proc. 2015;2:3580–3585. [Google Scholar]
- 284.Takahashi M., Kikuchi M., Takada Y. Mechanical properties and microstructures of dental cast Ti-6Nb-4Cu, Ti-18Nb-2Cu, and Ti-24Nb-1Cu alloys. Dent. Mater. J. 2016;35:564–570. doi: 10.4012/dmj.2015-354. [DOI] [PubMed] [Google Scholar]
- 285.Zhang B.B. Harbin Engineering University; Harbin: 2011. Microstructure and Biocompatibility of Ti-Ag Based Alloys. [Google Scholar]
- 286.Aoki T., Okafor I.C.I., Watanabe I., Hattori M., Oda Y., Okabe T. Mechanical properties of cast Ti-6Al-4V-xCu alloys. J. Oral Rehabil. 2004;31:1109–1114. doi: 10.1111/j.1365-2842.2004.01347.x. [DOI] [PubMed] [Google Scholar]
- 287.Jovanović M.T., Tadić S., Zec S., Mišković Z., Bobić I. The effect of annealing temperatures and cooling rates on microstructure and mechanical properties of investment cast Ti–6Al–4V alloy. Mater. Des. 2006;27:192–199. [Google Scholar]
- 288.Zhang E.L., Li S.Y., Ren J., Zhang L., Han Y. Effect of extrusion processing on the microstructure, mechanical properties, biocorrosion properties and antibacterial properties of Ti-Cu sintered alloys. Mater. Sci. Eng. C. 2016;69:760–768. doi: 10.1016/j.msec.2016.07.051. [DOI] [PubMed] [Google Scholar]
- 289.Kikuchi M., Takahashi M., Okuno O. Elastic moduli of cast Ti-Au, Ti-Ag, and Ti-Cu alloys. Dent. Mater. 2006;22:641–646. doi: 10.1016/j.dental.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 290.Cai D.G., Zhao X.T., Wang R.X., Qin G.W., Chen D.F., Zhang E.L. A novel biomedical titanium alloy with high antibacterial property and low elastic modulus. J. Mater. Sci. Technol. 2021;81:13–25. [Google Scholar]
- 291.Kikuchi M., Takada Y., Kiyosue S., Yoda M., Woldu M., Cai Z. Grindability of cast Ti-Cu alloys. Dent. Mater. 2003;19:375–381. doi: 10.1016/s0109-5641(02)00080-5. [DOI] [PubMed] [Google Scholar]
- 292.Koike M., Cai Z., Oda Y., Hattori M., Fujii H., Okabe T. Corrosion behavior of cast Ti-6Al-4V alloyed with Cu. J. Biomed. Mater. Res. 2005;73:368–374. doi: 10.1002/jbm.b.30225. [DOI] [PubMed] [Google Scholar]
- 293.Pina V.G., Amigó V., Muñoz A.I. Microstructural, electrochemical and tribo-electrochemical characterisation of titanium-copper biomedical alloys. Corrosion Sci. 2016;109:115–125. [Google Scholar]
- 294.Wang S., Ma Z., Liao Z.H., Song J., Yang K., Liu W.Q. Study on improved tribological properties by alloying copper to CP-Ti and Ti-6Al-4V alloy. Mater. Sci. Eng. C. 2015;57:123–132. doi: 10.1016/j.msec.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 295.Akbarpour M.R., Javadhesari S.M. Wear performance of novel nanostructured Ti-Cu intermetallic alloy as a potential material for biomedical applications. J. Alloys Compd. 2017;699:882–886. [Google Scholar]
- 296.Ottria L., Lauritano D., Bassi M.A., Palmieri A., Candotto V. Mechanical, chemical and biological aspects of titanium and titanium alloys in implant dentistry. J. Biol. Regul. Homeost. Agents. 2018;32:81–90. [PubMed] [Google Scholar]
- 297.Osório W.R., Cremasco A., Andrade P.N., Garcia A., Caram R. Electrochemical behavior of centrifuged cast and heat treated Ti-Cu alloys for medical applications. Electrochim. Acta. 2010;55:759–770. [Google Scholar]
- 298.Zhang B.B., Zheng Y.F., Liu Y. Effect of Ag on the corrosion behavior of Ti–Ag alloys in artificial saliva solutions. Dent. Mater. 2009;25:672–677. doi: 10.1016/j.dental.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 299.Takahashi M., Kikuchi M., Takada Y., Okabe T., Okuno O. Electrochemical behavior of cast Ti-Ag alloys. Dent. Mater. 2006;25:516–523. doi: 10.4012/dmj.25.516. [DOI] [PubMed] [Google Scholar]
- 300.Takada Y., Nakajima H., Okuno O., Okabe T. Microstructure and corrosion behavior of binary titanium alloys with beta-stabilizing elements. Dent. Mater. 2001;20:34–52. doi: 10.4012/dmj.20.34. [DOI] [PubMed] [Google Scholar]
- 301.Wei H., Wei Y.H., Hou L.F., Dang N. Correlation of ageing precipitates with the corrosion behaviour of Cu-4wt.% Ti alloys in 3.5wt.% NaCl solution. Corrosion Sci. 2016;111:382–390. [Google Scholar]
- 302.Ramezanzadeh B., Attar M.M. Studying the effects of micro and nano sized ZnO particles on the corrosion resistance and deterioration behavior of an epoxy-polyamide coating on hot-dip galvanized steel. Prog. Org. Coating. 2011;71:314–328. [Google Scholar]
- 303.Seah K.H.W., Thampuran R., Chen X., Teoh S.H. A comparison between the coorrosion behaviour of sintered and unsintered porous titanium. Corrosion Sci. 1995;37:1333–1340. [Google Scholar]
- 304.Ma Z., Li M., Liu R., Ren L., Zhang Y., Pan H.B. In vitro study on an antibacterial Ti-5Cu alloy for medical application. J. Mater. Sci. Mater. Med. 2016;27 doi: 10.1007/s10856-016-5698-1. [DOI] [PubMed] [Google Scholar]
- 305.Rolla G., Saxegaard E. Critical evaluation of the composition and use of topical fluorides, with emphasis on the role of calcium fluoride in caries inhibition. Dent Res. 1990;69:780–785. doi: 10.1177/00220345900690S150. [DOI] [PubMed] [Google Scholar]
- 306.Stookey G.K. Critical evaluation of the composition and use of topical fluorides. Dent Res. 1990;69:805–812. doi: 10.1177/00220345900690S154. [DOI] [PubMed] [Google Scholar]
- 307.Reclaru L., Meyer J.M. Effects of fluorides on titanium and other dental alloys in dentistry. 1998;19:85–92. doi: 10.1016/s0142-9612(97)00179-8. [DOI] [PubMed] [Google Scholar]
- 308.Nakagawa M., Matsuya S., Shiraishi T., Ohta M. Effect of fluoride concentration and pH on corrosion behavior of titanium for dental use. J. Dent. Res. 1999;78:1568–1572. doi: 10.1177/00220345990780091201. [DOI] [PubMed] [Google Scholar]
- 309.Shim H.M., Oh K.T., Woo J.Y., Hwang C.J., Kim K.M. Corrosion Resistance of titanium-silver alloys in an artificial saliva containing fluoride ions. J. Biomed. Mater. Res. B Appl. Biomater. 2005;73B:252–259. doi: 10.1002/jbm.b.30206. [DOI] [PubMed] [Google Scholar]
- 310.Mutlu I. Electrochemical Corrosion Behavior of TiN-coated biomedical Ti-Cu alloy foam in fluoride containing artificial saliva. Metall. Mater. Trans. 2014;45:3640–3649. [Google Scholar]
- 311.Takahashi M., Kikuchi M., Takada Y. Corrosion behavior of Ti-Ag alloys used in dentistry in lactic acid solution. Met. Mater. Int. 2011;17:175–179. [Google Scholar]
- 312.Zhang E.L., Zheng L.L., Liu J., Bai B., Liu C. Influence of Cu content on the cell biocompatibility of Ti-Cu sintered alloys. Mater. Sci. Eng. C. 2015;46:148–157. doi: 10.1016/j.msec.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 313.Li Y., Zhang Y., He J., Ma K., Wang M., Li S. Effects of different copper contents on the adhesion and migration of osteoblasts. Chin. J. Pract. Stomatol. 2017;10:354–359. [Google Scholar]
- 314.Tang Z.B., Niu J.L., Huang H., Zhang H., Pei J., Ou J.M. Potential biodegradable Zn-Cu binary alloys developed for cardiovascular implant applications. J. Mech. Behav. Biomed. Mater. 2017;72:182–191. doi: 10.1016/j.jmbbm.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 315.Xie Y., Zhao L.C., Zhang Z., Wang X., Wang R., Cui C.X. Fabrication and properties of porous Zn-Ag alloy scaffolds as biodegradable materials. Mater. Chem. Phys. 2018;219:433–443. [Google Scholar]
- 316.Yue R., Niu J.L., Li Y.T., Ke G.Z., Huang H., Pei J. In vitro cytocompatibility, hemocompatibility and antibacterial properties of biodegradable Zn-Cu-Fe alloys for cardiovascular stents applications. Mater. Sci. Eng. C. 2020;113:111007. doi: 10.1016/j.msec.2020.111007. [DOI] [PubMed] [Google Scholar]
- 317.Xiao C., Wang L.Q., Ren Y.P., Sun S.N., Zhang E.L., Yan C.N. Indirectly extruded biodegradable Zn-0.05wt%Mg alloy with improved strength and ductility: in vitro and in vivo studies. J. Mater. Sci. Technol. 2018;34:1618–1627. [Google Scholar]
- 318.Lin J.X., Tong X., Shi Z.M., Zhang D.C., Zhang L.S., Wang K. A biodegradable Zn-1Cu-0.1Ti alloy with antibacterial properties for orthopedic applications. Acta Biomater. 2020;106:410–427. doi: 10.1016/j.actbio.2020.02.017. [DOI] [PubMed] [Google Scholar]
- 319.Zhang W.T., Li P., Shen G., Mo X.S., Zhou C., Alexander D. Appropriately adapted properties of hot-extruded Zn-0.5Cu-xFe alloys aimed for biodegradable guided bone regeneration membrane application. Bioact. Mater. 2021;6:975–989. doi: 10.1016/j.bioactmat.2020.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Bakhsheshi-Rad H.R., Hamzah E., Low H.T., Kasiri-Asgarani M., Farahany S., Akbari E. Fabrication of biodegradable Zn-Al-Mg alloy: mechanical properties, corrosion behavior, cytotoxicity and antibacterial activities. Mater. Sci. Eng. C. 2017;73:215–219. doi: 10.1016/j.msec.2016.11.138. [DOI] [PubMed] [Google Scholar]
- 321.Shi Z.Z., Yu J., Liu X.F., Zhang H.J., Zhang D.W., Yin Y.X. Effects of Ag, Cu or Ca addition on microstructure and comprehensive properties of biodegradable Zn-0.8Mn alloy. Mater. Sci. Eng. C. 2019;99:969–978. doi: 10.1016/j.msec.2019.02.044. [DOI] [PubMed] [Google Scholar]
- 322.Niu J.L., Tang Z.B., Huang H., Pei J., Zhang H., Yuan G.Y. Research on a Zn-Cu alloy as a biodegradable material for potential vascular stents application. Mater. Sci. Eng. C. 2016;69:407–413. doi: 10.1016/j.msec.2016.06.082. [DOI] [PubMed] [Google Scholar]
- 323.Li P., Schille C., Schweizer E., Rupp F., Heiss A., Legner C. Mechanical characteristics, in vitro degradation, cytotoxicity, and antibacterial evaluation of Zn-4.0Ag alloy as a biodegradable material. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19030755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Katarivas Levy G., Goldman J., Aghion E. The prospects of zinc as a structural material for biodegradable implants—a review paper. Metals. 2017;7:402. [Google Scholar]
- 325.Sikora-Jasinska M., Mostaed E., Mostaed A., Beanland R., Mantovani D., Vedani M. Fabrication, mechanical properties and in vitro degradation behavior of newly developed ZnAg alloys for degradable implant applications. Mater. Sci. Eng. C. 2017;77:1170–1181. doi: 10.1016/j.msec.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 326.Vojtěch D., Kubásek J., Šerák J., Novák P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater. 2011;7:3515–3522. doi: 10.1016/j.actbio.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 327.Kubásek J., Vojtěch D., Jablonská E., Pospíšilová I., Lipov J., Ruml T. Structure, mechanical characteristics and in vitro degradation, cytotoxicity, genotoxicity and mutagenicity of novel biodegradable Zn-Mg alloys. Mater. Sci. Eng. C. 2016;58:24–35. doi: 10.1016/j.msec.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 328.Gong H., Wang K., Strich R., Zhou J.G. In vitro biodegradation behavior, mechanical properties, and cytotoxicity of biodegradable Zn-Mg alloy. J. Biomed. Mater. Res. B Appl. Biomater. 2015;103:1632–1640. doi: 10.1002/jbm.b.33341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Mostaed E., Sikora-Jasinska M., Mostaed A., Loffredo S., Demir A.G., Previtali B. Novel Zn-based alloys for biodegradable stent applications: design, development and in vitro degradation. J. Mech. Behav. Biomed. Mater. 2016;60:581–602. doi: 10.1016/j.jmbbm.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 330.Li H.F., Xie X.H., Zheng Y.F., Cong Y., Zhou F.Y., Qiu K.J. Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr. Sci. Rep. 2015;5:10719. doi: 10.1038/srep10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Ma D., Li Y., Ng S.C., Jones H. Unidirectional solidification of Zn-rich Zn-Cu peritectic alloys—I. Microstructure selection. Acta Mater. 2000;48:419–431. [Google Scholar]
- 332.Yao C., Wang Z., Tay S.L., Zhu T., Gao W. Effects of Mg on microstructure and corrosion properties of Zn-Mg alloy. J. Alloys Compd. 2014;602:101–107. [Google Scholar]
- 333.Liu X.W., Sun J.K., Zhou F.Y., Yang Y.H., Chang R.C., Qiu K.J. Micro-alloying with Mn in Zn-Mg alloy for future biodegradable metals application. Mater. Des. 2016;94:95–104. [Google Scholar]
- 334.Sun S.N., Ren Y.P., Wang L.Q., Yang B., Li H.X., Qin G.W. Abnormal effect of Mn addition on the mechanical properties of as-extruded Zn alloys. Mater. Sci. Eng. A. 2017;701:129–133. [Google Scholar]
- 335.Kabir H., Munir K., Wen C., Li Y. Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: biomechanical and biocorrosion perspectives. Bioact. Mater. 2021;6:836–879. doi: 10.1016/j.bioactmat.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Pachla W., Przybysz S., Jarzębska A., Bieda M., Sztwiertnia K., Kulczyk M. Structural and mechanical aspects of hypoeutectic Zn-Mg binary alloys for biodegradable vascular stent applications. Bioact. Mater. 2021;6:26–44. doi: 10.1016/j.bioactmat.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.An S.F., Gong Q.M., Huang Y.H. Promotive effect of zinc ions on the vitality, migration, and osteogenic differentiation of human dental pulp cells. Biol. Trace Elem. Res. 2017;175:112–121. doi: 10.1007/s12011-016-0763-7. [DOI] [PubMed] [Google Scholar]
- 338.Qiao Y.P., Zhang W.J., Tian P., Meng F.H., Zhu H.Q., Jiang X.Q. Stimulation of bone growth following zinc incorporation into biomaterials. Biomaterials. 2014;35:6882–6897. doi: 10.1016/j.biomaterials.2014.04.101. [DOI] [PubMed] [Google Scholar]
- 339.Ma J., Zhao N., Zhu D.H. Endothelial cellular responses to biodegradable metal zinc. ACS Biomater. Sci. Eng. 2015;1:1174–1182. doi: 10.1021/acsbiomaterials.5b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Wang J.L., Witte F., Xi T.F., Zheng Y.F., Yang K., Yang Y.S. Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomater. 2015;21:237–249. doi: 10.1016/j.actbio.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 341.Bowen P.K., Guillory R.J., 2nd, Shearier E.R., Seitz J.-M., Drelich J., Bocks M. Metallic zinc exhibits optimal biocompatibility for bioabsorbable endovascular stents. Mater. Sci. Eng. C. 2015;56:467–472. doi: 10.1016/j.msec.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Yang H.T., Wang C., Liu C.Q., Chen H.W., Wu Y.F., Han J.T. Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model. Biomaterials. 2017;145:92–105. doi: 10.1016/j.biomaterials.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 343.Staiger M.P., Pietak A.M., Huadmai J., Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006;27:1728–1734. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 344.Sezer N., Evis Z., Kayhan S.M., Tahmasebifar A., Koç M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloys. 2018;6:23–43. [Google Scholar]
- 345.Robinson D.A., Griffith R.W., Shechtman D., Evans R.B., Conzemius M.G. In vitro antibacterial properties of magnesium metal against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Acta Biomater. 2010;6:1869–1877. doi: 10.1016/j.actbio.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 346.Ren L., Lin X., Tan L.L., Yang K. Effect of surface coating on antibacterial behavior of magnesium based metals. Mater. Lett. 2011;65:3509–3511. [Google Scholar]
- 347.Lock J.Y., Wyatt E., Upadhyayula S., Whall A., Nunez V., Vullev V.I. Degradation and antibacterial properties of magnesium alloys in artificial urine for potential resorbable ureteral stent applications. J. Biomed. Mater. Res. 2014;102A:781–792. doi: 10.1002/jbm.a.34741. [DOI] [PubMed] [Google Scholar]
- 348.Nan L., Xu D.K., Gu T.Y., Song X., Yang K. Microbiological influenced corrosion resistance characteristics of a 304L-Cu stainless steel against Escherichia coli. Mater. Sci. Eng. C. 2015;48:228–234. doi: 10.1016/j.msec.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 349.Witte F., Fischer J., Nellesen J., Crostack H.A., Kaese V., Pisch A. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials. 2006;27:1013–1018. doi: 10.1016/j.biomaterials.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 350.Hou P., Zhao C.L., Cheng P.F., Wu H.L., Ni J.H., Zhang S.X. Reduced antibacterial property of metallic magnesium in vivo. Biomed. Mater. 2016;12 doi: 10.1088/1748-605X/12/1/015010. [DOI] [PubMed] [Google Scholar]
- 351.Li Y., Liu G.W., Zhai Z.J., Liu L.N., Li H.W., Yang K. Antibacterial properties of magnesium in vitro and in an in vivo model of implant-associated methicillin-resistant Staphylococcus aureus infection. Antimicrob. Agents Chemother. 2014;58:7586–7591. doi: 10.1128/AAC.03936-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Rahim M.I., Eifler R., Rais B., Mueller P.P. Alkalization is responsible for antibacterial effects of corroding magnesium. J. Biomed. Mater. Res. A. 2015;103:3526–3532. doi: 10.1002/jbm.a.35503. [DOI] [PubMed] [Google Scholar]
- 353.Chen J., Peng W., Zhu L., Tan L., Etim I.P., Wang X. Effect of copper content on the corrosion behaviors and antibacterial properties of binary Mg-Cu alloys. Mater. Technol. 2018;33:145–152. [Google Scholar]
- 354.He G.P., Wu Y.H., Zhang Y., Zhu Y., Liu Y., Li N. Addition of Zn to the ternary Mg-Ca-Sr alloys significantly improves their antibacterial properties. J. Mater. Chem. B. 2015;3:6676–6689. doi: 10.1039/C5TB01319D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Qin H., Zhao Y.C., An Z.Q., Cheng M.Q., Wang Q., Cheng T. Enhanced antibacterial properties, biocompatibility, and corrosion resistance of degradable Mg-Nd-Zn-Zr alloy. Biomaterials. 2015;53:211–220. doi: 10.1016/j.biomaterials.2015.02.096. [DOI] [PubMed] [Google Scholar]
- 356.Gao Z.H., Song M.S., Liu R.L., Shen Y.S., Ward L., Cole I. Improving in vitro and in vivo antibacterial functionality of Mg alloys through micro-alloying with Sr and Ga. Mater. Sci. Eng. C. 2019;104:109926. doi: 10.1016/j.msec.2019.109926. [DOI] [PubMed] [Google Scholar]
- 357.Liu C. Nanjing University of Science & Technology; 2016. Design and Biomedical Function of New Degradable Magnesium Alloy for Bone Implantation. [Google Scholar]
- 358.Liu C., Fu X.K., Pan H.B., Wan P., Wang L., Tan L.L. Biodegradable Mg-Cu alloys with enhanced osteogenesis, angiogenesis, and long-lasting antibacterial effects. Sci. Rep. 2016;6 doi: 10.1038/srep27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Zhang C., Lin J., Nguyen N.Y.T., Guo Y., Xu C., Seo C. Antimicrobial bioresorbable Mg-Zn-Ca alloy for bone repair in a comparison study with Mg-Zn-Sr alloy and pure Mg. ACS Biomater. Sci. Eng. 2019;6:517–538. doi: 10.1021/acsbiomaterials.9b00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Liu Z., Schade R., Luthringer B., Hort N., Rothe H., Muller S. Influence of the microstructure and silver content on degradation, cytocompatibility, and antibacterial properties of magnesium-silver alloys in vitro. Oxid Med Cell Longev. 2017;2017:8091265. doi: 10.1155/2017/8091265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Brooks E.K., Ahn R., Tobias M.E., Hansen L.A., Luke-Marshall N.R., Wild L. Magnesium alloy AZ91 exhibits antimicrobial properties in vitro but not in vivo. J. Biomed. Mater. Res. B. 2018;106:221–227. doi: 10.1002/jbm.b.33839. [DOI] [PubMed] [Google Scholar]
- 362.Yan X.D., Wan P., Tan L.L., Zhao M.C., Qin L., Yang K. Corrosion and biological performance of biodegradable magnesium alloys mediated by low copper addition and processing. Mater. Sci. Eng. C. 2018;93:565–581. doi: 10.1016/j.msec.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 363.Wang Z., Yang J., Li J.J. Antibacterial properties of new magnesium alloy implanted in vivo. Res. Tissue Eng. China. 2018;22:3451–3455. [Google Scholar]
- 364.Razzaghi M., Kasiri-Asgarani M., Bakhsheshi-Rad H.R., Ghayour H. In vitro degradation, antibacterial activity and cytotoxicity of Mg-3Zn-xAg nanocomposites synthesized by mechanical alloying for implant applications. J. Mater. Eng. Perform. 2019;28:1441–1455. [Google Scholar]
- 365.Li S. Zhengzhou University; Henan: 2016. Study on the Tissue and Antibacterial Properties of Degradable Mg-Zn-Y-Nd-Ag Alloy for Bone Implantation. [Google Scholar]
- 366.Qu C.C. Zhengzhou University; Henan: 2017. Microstructure and Properties of Extruded Mg-Zn-Y-Nd-Xcu Alloy for Biological Use. [Google Scholar]
- 367.Bakhsheshi-Rad H.R., Dayaghi E., Ismail A.F., Aziz M., Akhavan-Farid A., Chen X. Synthesis and in-vitro characterization of biodegradable porous magnesium-based scaffolds containing silver for bone tissue engineering. Trans. Nonferrous Metals Soc. China. 2019;29:984–996. [Google Scholar]
- 368.Cai S.H., Lei T., Li N.F., Feng F.F. Effects of Zn on microstructure, mechanical properties and corrosion behavior of Mg-Zn alloys. Mater. Sci. Eng. C. 2012;32:2570–2577. [Google Scholar]
- 369.Tie D., Feyerabend F., Hort N., Hoeche D., Kainer K.U., Willumeit R. In vitro mechanical and corrosion properties of biodegradable Mg-Ag alloys. Mater. Corrosion-Werkstoffe Und Korrosion. 2014;65:569–576. [Google Scholar]
- 370.Clark J.B. Transmission electron microscopy study of age hardening in a Mg-5 wt.% Zn alloy. Acta Metall. 1965;13:1281–1289. [Google Scholar]
- 371.Buha J. Reduced temperature (22-100°C) ageing of an Mg-Zn alloy. Mater. Sci. Eng. A. 2008;492:11–19. [Google Scholar]
- 372.Shi Z.M., Song G.L., Atrens A. Corrosion resistance of anodised single-phase Mg alloys. Surf. Coating. Technol. 2006;201:492–503. [Google Scholar]
- 373.Liu X.B., Shan D.Y., Song Y.W., Han E.H. Effects of heat treatment on corrosion behaviors of Mg-3Zn magnesium alloy. Trans. Nonferrous Metals Soc. China. 2010;20:1345–1350. [Google Scholar]
- 374.Yin P., Li N.F., Lei T., Liu L., Ouyang C. Effects of Ca on microstructure, mechanical and corrosion properties and biocompatibility of Mg-Zn-Ca alloys. J. Mater. Sci. Mater. Med. 2013;24:1365–1373. doi: 10.1007/s10856-013-4856-y. [DOI] [PubMed] [Google Scholar]
- 375.Berglund I.S., Brar H.S., Dolgova N., Acharya A.P., Keselowsky B.G., Sarntinoranont M. Synthesis and characterization of Mg-Ca-Sr alloys for biodegradable orthopedic implant applications. J. Biomed. Mater. Res. B. 2012;100:1524–1534. doi: 10.1002/jbm.b.32721. [DOI] [PubMed] [Google Scholar]
- 376.Zhen Z., Liu X.L., Huang T., Xi T.F., Zheng Y.F. Hemolysis and cytotoxicity mechanisms of biodegradable magnesium and its alloys. Mater. Sci. Eng. C. 2015;46:202–206. doi: 10.1016/j.msec.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 377.Fischer J., Pröfrock D., Hort N., Willumeit R., Feyerabend F. Reprint of: improved cytotoxicity testing of magnesium materials. Mater. Sci. Eng., B. 2011;176:1773–1777. [Google Scholar]
- 378.Sun Y., Wu H.L., Wang W.H., Zan R., Peng H.Z., Zhang S.X. Translational status of biomedical Mg devices in China. Bioact. Mater. 2019;4:358–365. doi: 10.1016/j.bioactmat.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Rahim M.I., Rohde M., Rais B., Seitz J.M., Mueller P.P. Susceptibility of metallic magnesium implants to bacterial biofilm infections. J. Biomed. Mater. Res. A. 2016;104:1489–1499. doi: 10.1002/jbm.a.35680. [DOI] [PubMed] [Google Scholar]
- 380.Wang S., Yang C.G., Ren L., Shen M.G., Yang K. Study on antibacterial performance of Cu-bearing cobalt-based alloy. Mater. Lett. 2014;129:88–90. [Google Scholar]
- 381.Jiang F.L., Zhu W.W., Zhao C.C., Li Y.L., Ren F.Z. A strong, wear- and corrosion-resistant, and antibacterial Co-30 at.% Cr-5 at.% Ag ternary alloy for medical implants. Mater. Des. 2019;184:108190. [Google Scholar]
- 382.Hosman A.H., Mei HCvd, Bulstra S.K., Kuijer R., Busscher H.J., Neut D. Influence of Co-Cr particles and Co-Cr ions on the growth of staphylococcal biofilms. Int. J. Artif. Organs. 2011;34:759–765. doi: 10.5301/ijao.5000031. [DOI] [PubMed] [Google Scholar]
- 383.Ren L., Memarzadeh K., Zhang S., Sun Z., Yang C., Ren G. A novel coping metal material CoCrCu alloy fabricated by selective laser melting with antimicrobial and antibiofilm properties. Mater. Sci. Eng. C. 2016;67:461–467. doi: 10.1016/j.msec.2016.05.069. [DOI] [PubMed] [Google Scholar]
- 384.Lu Y.J., Ren L., Xu X.C., Yang Y., Wu S.Q., Luo J.S. Effect of Cu on microstructure, mechanical properties, corrosion resistance and cytotoxicity of CoCrW alloy fabricated by selective laser melting. J. Mech. Behav. Biomed. Mater. 2018;81:130–141. doi: 10.1016/j.jmbbm.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 385.Lu Y.J., Zhao C.Q., Ren L., Guo S., Gan Y.L., Yang C.G. Preliminary assessment of metal-porcelain bonding strength of CoCrW alloy after 3 wt.% Cu addition. Mater. Sci. Eng. C. 2016;63:37–45. doi: 10.1016/j.msec.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 386.Wang R.X., Wang R.X., Chen D.F., Qin G.W., Zhang E.L. Novel CoCrWNi alloys with Cu addition: microstructure, mechanical properties, corrosion properties and biocompatibility. J. Alloys Compd. 2020;824:153924. [Google Scholar]
- 387.Wang R.X., Qin G.W., Zhang E.L. Effect of Cu on Martensite Transformation of CoCrMo alloy for biomedical application. J. Mater. Sci. Technol. 2020;52:127–135. [Google Scholar]
- 388.Barceloux D.G. Cobalt. J. Toxicol. Clin. Toxicol. 1999;37:201–206. doi: 10.1081/clt-100102420. [DOI] [PubMed] [Google Scholar]
- 389.Paustenbach D.J., Tvermoes B.E., Unice K.M., Finley B.L., Kerger B.D. A review of the health hazards posed by cobalt. Crit. Rev. Toxicol. 2013;43:316–362. doi: 10.3109/10408444.2013.779633. [DOI] [PubMed] [Google Scholar]
- 390.Tvermoes B.E., Paustenbach D.J., Kerger B.D., Finley B.L., Unice K.M. Review of cobalt toxicokinetics following oral dosing: implications for health risk assessments and metal-on-metal hip implant patients. Crit. Rev. Toxicol. 2015;45:367–387. doi: 10.3109/10408444.2014.985818. [DOI] [PubMed] [Google Scholar]
- 391.Pettersson M., Johansson A., Thorén M.M. Inflammatory response by Ti, Co-Cr-Mo in cultures of human macrophages. Dent. Mater. 2012;28(Supplement 1):e32. [Google Scholar]
- 392.Grass G., Rensing C., Solioz M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011;77:1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Wilks S.A., Michels H., Keevil C.W. The survival of Escherichia coli O157 on a range of metal surfaces. Int. J. Food Microbiol. 2005;105:445–454. doi: 10.1016/j.ijfoodmicro.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 394.Michels H.T., Wilks S.A., Noyce J.O., Keevil C.W. Material Science and Technology Conference, Editor. Copper for the 21st Century Symposium. 2005. Copper alloys for human infectious disease control. Pittsburgh, PA. September 25-28. [Google Scholar]
- 395.Mehtar S., Wiid I., Todorov S.D. The antimicrobial activity of copper and copper alloys against nosocomial pathogens and Mycobacterium tuberculosis isolated from healthcare facilities in the Western Cape: an in-vitro study. J. Hosp. Infect. 2008;68:45–51. doi: 10.1016/j.jhin.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 396.Yousuf B., Ahire J.J., Dicks L.M.T. Understanding the antimicrobial activity behind thin- and thick-rolled copper plates. Appl. Microbiol. Biotechnol. 2016;100:5569–5580. doi: 10.1007/s00253-016-7361-7. [DOI] [PubMed] [Google Scholar]
- 397.Marais F., Mehtar S., Chalkley L. Antimicrobial efficacy of copper touch surfaces in reducing environmental bioburden in a South African community healthcare facility. J. Hosp. Infect. 2010;74:80–82. doi: 10.1016/j.jhin.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 398.Mikolay A., Huggett S., Tikana L., Grass G., Braun J., Nies D.H. Survival of bacteria on metallic copper surfaces in a hospital trial. Appl. Microbiol. Biotechnol. 2010;87:1875–1879. doi: 10.1007/s00253-010-2640-1. [DOI] [PubMed] [Google Scholar]
- 399.Palza H., Nuñez M., Bastías R., Delgado K. In situ antimicrobial behavior of materials with copper-based additives in a hospital environment. Int. J. Antimicrob. Agents. 2018;51:912–917. doi: 10.1016/j.ijantimicag.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 400.Borkow G., Gabbay J. Copper, an ancient remedy returning to fight microbial, fungal and viral infections. Curr. Chem. Biol. 2009;3:272–278. [Google Scholar]
- 401.Santo C.E., Morais P.V., Grass G. Isolation and characterization of bacteria resistant to metallic copper surfaces. Appl. Environ. Microbiol. 2010;76:1341–1348. doi: 10.1128/AEM.01952-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Demetriou M.D., Wiest A., Hofmann D.C., Johnson W.L., Han B., Wolfson N. Amorphous metals for hard-tissue prosthesis. JOM. 2010;62:83–91. [Google Scholar]
- 403.Buzzi S., Jin K., Uggowitzer P.J., Tosatti S., Gerber I., Löffler J.F. Cytotoxicity of Zr-based bulk metallic glasses. Intermetallics. 2006;14:729–734. [Google Scholar]
- 404.Sun Y., Huang Y., Fan H., Wang Y., Ning Z., Liu F. In vitro and in vivo biocompatibility of an Ag-bearing Zr-based bulk metallic glass for potential medical use. J. Non-Cryst. Solids. 2015;419:82–91. [Google Scholar]
- 405.Horton J., Parsell D. Biomedical potential of a zirconium-based bulk metallic glass. MRS Proc. 2011:754. [Google Scholar]
- 406.Lin B., Mu R., Yang L., Bian X. Antibacterial effect of metallic glasses. Chin. Sci. Bull. 2012;57:1069–1072. [Google Scholar]
- 407.Latuch J., Błyskun P., Kulik T. Wydawnictwo SIGMA-NOT; 2012. Influence of Silver on the Glass Forming Ability and Mechanical Properties in Cu-Zr-Ti-Ag Bulk Metallic Glasses. [Google Scholar]
- 408.Lesz S., Babilas R., Nowosielski R. Influence of copper addition on glass forming ability, thermal stability, structure and magnetic properties of Fe-Co-Based BMGs. Solid State Phenom. 2013;203–204:296–301. [Google Scholar]
- 409.Chu J.P., Jang J.S.C., Huang J.C., Chou H.S., Yang Y., Ye J.C. Thin film metallic glasses: unique properties and potential applications. Thin Solid Films. 2012;520:5097–5122. [Google Scholar]
- 410.Chiang P.T., Chen G.J., Jian S.R., Shih Y.H., Jang J.S.C., Lai C.H. Surface antimicrobial effects of Zr61Al7.5Ni10Cu17.5Si4 thin film metallic glasses on Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, acinetobacter baumannii and Candida albicans. Fooyin Journal of Health Sciences. 2010;2:12–20. [Google Scholar]
- 411.Chu J.P., Liu T.Y., Li C.L., Wang C.H., Jang J.S.C., Chen M.J. Fabrication and characterizations of thin film metallic glasses: antibacterial property and durability study for medical application. Thin Solid Films. 2014;561:102–107. [Google Scholar]
- 412.Chen H.W., Hsu K.C., Chan Y.C., Duh J.G., Lee J.W., Jang J.S.C. Antimicrobial properties of Zr-Cu-Al-Ag thin film metallic glass. Thin Solid Films. 2014;561:98–101. [Google Scholar]
- 413.Subramanian B., Maruthamuthu S., Rajan S.T. Biocompatibility evaluation of sputtered zirconium-based thin film metallic glass-coated steels. Int. J. Nanomed. 2015;10(Suppl 1):17–29. doi: 10.2147/IJN.S79977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Liu Y., Padmanabhan J., Cheung B., Liu J.B., Chen Z., Scanley B.E. Combinatorial development of antibacterial Zr-Cu-Al-Ag thin film metallic glasses. Sci. Rep. 2016;6 doi: 10.1038/srep26950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Tsai P.H., Lin Y.Z., Li J.B., Jian S.R., Jang J.S.C., Li C. Sharpness improvement of surgical blade by means of ZrCuAlAgSi metallic glass and metallic glass thin film coating. Intermetallics. 2012;31:127–131. [Google Scholar]
- 416.Apreutesei M., Steyer P., Joly-Pottuz L., Billard A., Qiao J., Cardinal S. Microstructural, thermal and mechanical behavior of co-sputtered binary Zr-Cu thin film metallic glasses. Thin Solid Films. 2014;561:53–59. [Google Scholar]
- 417.Etiemble A., Der Loughian C., Apreutesei M., Langlois C., Cardinal S., Pelletier J.M. Innovative Zr-Cu-Ag thin film metallic glass deposed by magnetron PVD sputtering for antibacterial applications. J. Alloys Compd. 2017;707:155–161. [Google Scholar]
- 418.Zhang Y.M., Cheng Y.Q., Li W.C., Jie X.H. Effects of antibacterial heat treatment on cold workability of SUSXM7 Cu-bearing stainless steel. Hot Work. Technol. 2013;42:181–183. [Google Scholar]
- 419.Li K.Q., Xia C., Qiao Y.Q., Liu X.Y. Dose-response relationships between copper and its biocompatibility/antibacterial activities. J. Trace Elem. Med. Biol. 2019;55:127–135. doi: 10.1016/j.jtemb.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 420.Xiu Z.M., Ma J., Alvarez P.J.J. Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions. Environ. Sci. Technol. 2011;45:9003–9008. doi: 10.1021/es201918f. [DOI] [PubMed] [Google Scholar]
- 421.Quaranta D., Krans T., Santo C.E., Elowsky C.G., Domaille D.W., Chang C.J. Mechanisms of contact-mediated killing of yeast cells on dry metallic copper surfaces. Appl. Environ. Microbiol. 2011;77:416–426. doi: 10.1128/AEM.01704-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Padan E., Bibi E., Ito M., Krulwich T.A. Alkaline pH homeostasis in bacteria: new insights. Biochim. Biophys. Acta Biomembr. 2005;1717:67–88. doi: 10.1016/j.bbamem.2005.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Burne R.A., Marquis R.E. Alkali production by oral bacteria and protection against dental caries. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 2000;193:1–6. doi: 10.1111/j.1574-6968.2000.tb09393.x. [DOI] [PubMed] [Google Scholar]
- 424.Dong C.X., Cairney J., Sun Q.H., Maddan O.L., He G.H., Deng Y.L. Investigation of Mg(OH)2 nanoparticles as an antibacterial agent. J. Nanoparticle Res. 2010;12:2101–2109. [Google Scholar]
- 425.Jin T., He Y.P. Antibacterial activities of magnesium oxide (MgO) nanoparticles against foodborne pathogens. J. Nanoparticle Res. 2011;13:6877–6885. [Google Scholar]
- 426.Chernousova S., Epple M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013;52:1636–1653. doi: 10.1002/anie.201205923. [DOI] [PubMed] [Google Scholar]
- 427.Paladini F., Pollini M., Sannino A., Ambrosio L. Metal-based antibacterial substrates for biomedical applications. Biomacromolecules. 2015;16:1873–1885. doi: 10.1021/acs.biomac.5b00773. [DOI] [PubMed] [Google Scholar]
- 428.Faria AF de, Martinez D.S.T., Meira S.M.M., de Moraes A.C.M., Brandelli A., Filho A.G.S. Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids Surf. B Biointerfaces. 2014;113:115–124. doi: 10.1016/j.colsurfb.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 429.Li J.H., Wang G., Zhu H.Q., Zhang M., Zheng X.H., Di Z.F. Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer. Sci. Rep. 2014;4:4359. doi: 10.1038/srep04359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Li J.H., Zhou H.J., Qian S., Liu Z.W., Feng J.W., Jin P. Plasmonic gold nanoparticles modified titania nanotubes for antibacterial application. Appl. Phys. Lett. 2014;104 [Google Scholar]
- 431.Wang G.M., Jin W.H., Qasim A.M., Gao A., Peng X., Li W. Antibacterial effects of titanium embedded with silver nanoparticles based on electron-transfer-induced reactive oxygen species. Biomaterials. 2017;124:25–34. doi: 10.1016/j.biomaterials.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 432.Reece S.Y., Nocera D.G. Proton-coupled electron transfer in biology: results from synergistic studies in natural and model systems. Annu. Rev. Biochem. 2009;78:673–699. doi: 10.1146/annurev.biochem.78.080207.092132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Lovley D.R., Coates J.D., Blunt-Harris E.L., Phillips E.J.P., Woodward J.C. Humic substances as electron acceptors for microbial respiration. Nature. 1996;382:445–448. [Google Scholar]
- 434.Hernandez M.E., Newman D.K. Extracellular electron transfer. Cellular and Molecular Life Sciences CMLS. 2001;58:1562–1571. doi: 10.1007/PL00000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Lemire J.A., Harrison J.J., Turner R.J. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013;11:371. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 436.Zhang X.R., Yang C.G., Yang K. Contact killing of Cu-bearing stainless steel based on charge transfer caused by the microdomain potential difference. ACS Appl. Mater. Interfaces. 2020;12:361–372. doi: 10.1021/acsami.9b19596. [DOI] [PubMed] [Google Scholar]
- 437.Qiu W.J. Shanghai Jiaotong University; 2009. Process and Properties of Copper-Containing Austenite Antibacterial Stainless Steel. [Google Scholar]
- 438.van der Mei H.C., Atema-Smit J., Jager D., Langworthy D.E., Collias D.I., Mitchell M.D. Influence of adhesion to activated carbon particles on the viability of waterborne pathogenic bacteria under flow. Biotechnol. Bioeng. 2008;100:810–813. doi: 10.1002/bit.21820. [DOI] [PubMed] [Google Scholar]
- 439.Qi LF, Xu ZR, Jiang X, Hu CH, Zou XF. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 339:2693-27002004. [DOI] [PubMed]
- 440.Gottenbos B., Grijpma D.W., Busscher H.J., van der Mei H.C., Feijen J. Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria. J. Antimicrob. Chemother. 2001;48:7–13. doi: 10.1093/jac/48.1.7. [DOI] [PubMed] [Google Scholar]
- 441.Murata H., Koepsel R.R., Matyjaszewski K., Russell A.J. Permanent, non-leaching antibacterial surfaces—2: how high density cationic surfaces kill bacterial cells. Biomaterials. 2007;28:4870–4879. doi: 10.1016/j.biomaterials.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 442.Strahl H., Hamoen L.W. Membrane potential is important for bacterial cell division. Proc. Natl. Acad. Sci. U.S.A. 2010;107:12281–12286. doi: 10.1073/pnas.1005485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Zhang Y.M., Rock C.O. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 2008;6:222–233. doi: 10.1038/nrmicro1839. [DOI] [PubMed] [Google Scholar]
- 444.Santo C.E., Lam E.W., Elowsky C.G., Quaranta D., Domaille D.W., Chang C.J. Bacterial killing by dry metallic copper surfaces. Appl. Environ. Microbiol. 2011;77:794. doi: 10.1128/AEM.01599-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.