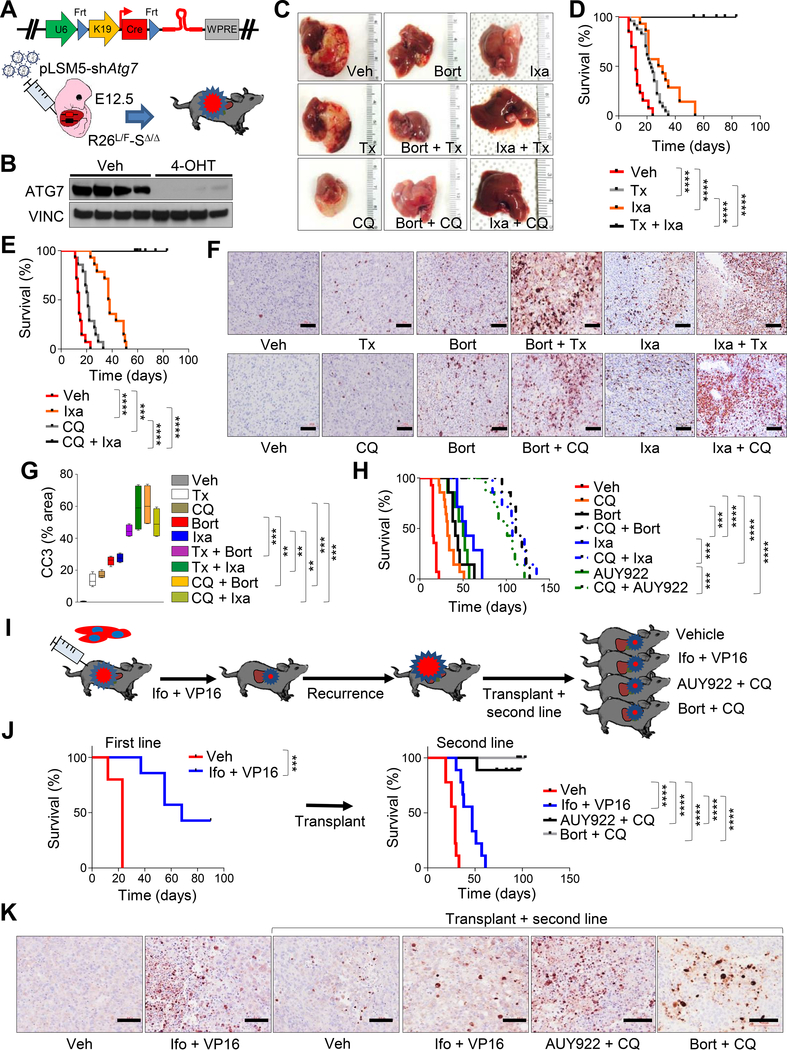
Figure 2. Pharmacological perturbation of UPR and suppression of autophagy induce tumor regression in murine MRTL.
A) Schematic model of experimental design for tumor maintenance studies. A bicistronic lentiviral vector expressing the Cre recombinase under the KRT19 promoter and a latent Flpo-inducible shRNA specific for Atg7 (pLSM5-shAtg7) was introduced in the liver of Rosa26LSL-Luc/FlpoERT2-Smarcb1LoxP/LoxP (R26L/F-SΔ/Δ) E12.5 embryos by trans-uterine injection to generate R26L/F-SΔ/Δ-pLSM5-shAtg7 mice. Tumor bearing mice were treated with tamoxifen to induce the Flpo-mediated activation of the latent shRNA. B) Western blot analysis of ATG7 levels in ex vivo cultures generated from the model in (A). Cell lysates were obtained after 96 hr of treatment with 4-OHT or vehicle. C) Assessment of tumor burden at necropsy in R26L/F-SΔ/Δ-pLSM5-shAtg7 mice treated with bortezomib, ixazomib, tamoxifen, chloroquine or the reported combinations. D-E) Kaplan-Meier survival analysis of tumor bearing mice assigned to single or combination treatments with ixazomib and tamoxifen ([D] vehicle: n = 13, tamoxifen: n = 23, ixazomib: n = 14, ixazomib + tamoxifen: n = 11) or ixazomib and chloroquine ([E] vehicle: n = 14, chloroquine: n = 14, ixazomib: n = 14, ixazomib + chloroquine: n = 10). F) Evaluation of apoptotic response in R26L/F-SΔ/Δ-pLSM5-shAtg7 tumor bearing mice treated for 7 days with the reported drugs. Apoptosis was assessed by Cleaved Caspase-3 (CC3) immunostaining. G) Quantification of apoptotic response in the experimental arms described above (n = 4 biological replicates/group). The percentage of apoptotic cells was expressed as CC3 positive area/total area of the section. Box and lines mark quartiles, ends of lines represent minimum and maximum. H) Kaplan-Meier survival analysis of Rag2−/− mice orthotopically transplanted with R26L/F-SΔ/Δ-pLSM5-shAtg7 tumors and assigned to single agents or combination treatments with ixazomib, bortezomib, NVP-AUY-922, and chloroquine (n = 7/group; vehicle and chloroquine: n = 14). I) Schematic model of experimental workflow for second line therapy. Orthotopic transplants of MRTL were treated with a combination of ifosfamide and etoposide (VP16) and monitored until tumor recurrence. Secondary transplants generated from terminal mice were assigned to second line therapy with the original drug combination or the bortezomib/chloroquine or NVP-AUY-922/chloroquine combinations. J) Left panel: Kaplan-Meier survival analysis for mice treated with first line chemotherapy regimen (n = 5/group). Right panel: Kaplan-Meier analysis of survival of secondary orthotopic transplants assigned to rescue therapy with the reported combinations (n = 9/group). K) Immunophenotypic assessment of apoptosis (Cleaved Caspase-3) in the treatment groups described above. Tumors were harvested for analysis after 7 days of treatment. ** p < 0.01, *** p < 0.001, **** p < 0.0001 by Mantel-Cox test (D, E, H, J) and by unpaired two-tailed t-test (G). Size bars: 100 μm. Veh: vehicle, Bort: bortezomib, Ixa: ixazomib, Tx: tamoxifen, 4-OHT: 4-hydroxytamoxifen, Ifo: ifosfamide, AUY922: NVP-AUY-922. See also Figure S2.