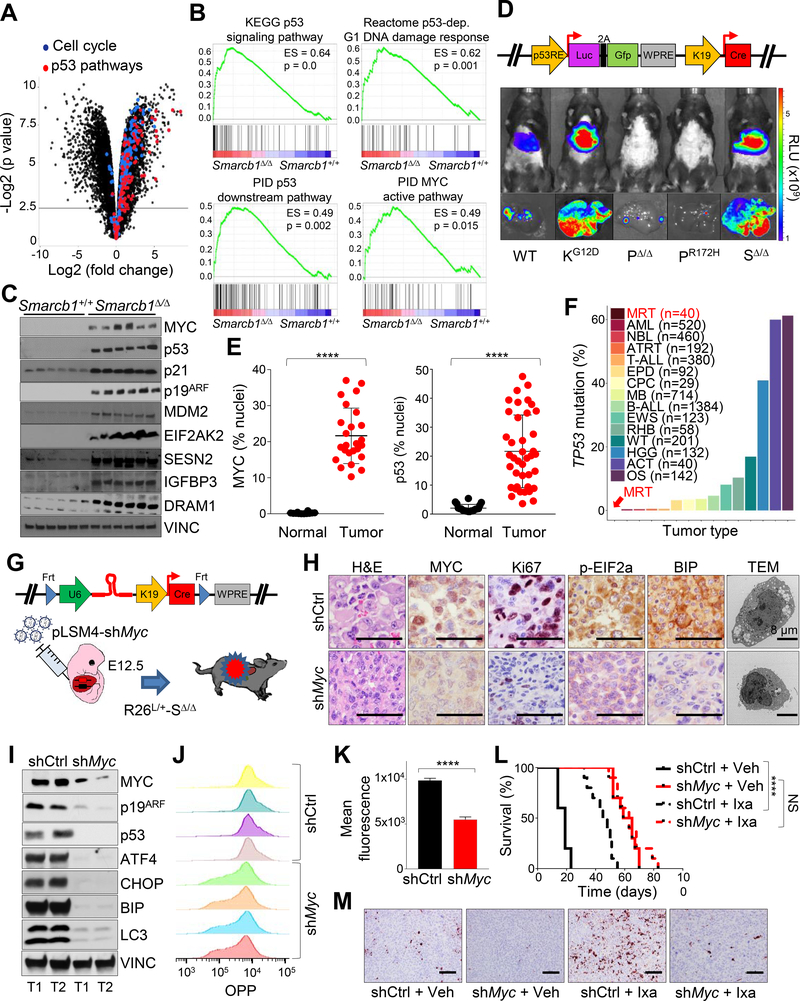
Figure 4. SMARCB1 loss drives autophagy through a MYC-p53 axis.
A) Volcano plot highlighting the dysregulation of cell cycle and p53 pathways in Smarcb1-ablated tumors. B) GSEA analysis for enrichment of p53 and MYC pathway targets in Smarcb1Δ/Δ tumors versus Smarcb1+/+ livers (n = 5 biological replicates/group). C) Western blot dissection of the MYC-p53 axis involved in cell cycle regulation and modulation of UPR and autophagy. D) Top panel: schematic showing the pLV-p53R-Cre bicistronic lentiviral reporter which allows for the in vivo, tissue-restricted expression of the Cre recombinase and visualization of p53 activity through a dual bioluminescence/fluorescence reporter. Bottom panels: activation of the luciferase reporter in Smarcb1LoxP/LoxP (SΔ/Δ) mice transduced at E12.5 and imaged at P90. A conditional KrasLSL-G12D (KG12D) allele was used as positive control. Trp53LoxP/LoxP (PΔ/Δ) and Trp53LSL-R172H/+ (PR172H) mice were used as negative controls. WT: wild-type. RLU: relative luminescence units. E) Quantification of MYC and p53 levels in human MRT and RMC samples (p53 normal: n = 20, p53 tumors: n = 40, MYC normal: n = 12, MYC tumor: n = 25). The levels of the nuclear proteins were expressed as percentage of stained nuclei/total nuclei in the section. F) Bar plots demonstrating the relative representation of somatic mutations in TP53 across pediatric human cancers (MRT: Malignat rhabdoid tumor, AML: Acute myeloid leukemia, NBL: Neuroblastoma, ATRT: Atypical teratoid rhabdoid tumor, T-ALL: T cell acute lymphoblastic leukemia, EPD: Ependymoma, CPC: Choroid plexus carcinoma, MB: Medulloblastoma, B-ALL: B cell acute lymphoblastic leukemia, EWS: Ewing’s sarcoma, RHB: Rhabdosarcoma, WT: Wilms tumor, HGG: High-grade glioma, ACT: Adrenocortical carcinoma, OS: Osteosarcoma) from different published studies (https://pecan.stjude.cloud) (Chun et al., 2016; Johann et al., 2016). G) Schematic model of experimental design to generate Myc hypomorphic tumors. A bicistronic lentiviral vector carrying the Cre recombinase under the KRT19 promoter and a constitutive shRNA specific for Myc (pLSM4-shMyc) or pLSM4-shCtrl were introduced in the liver of Rosa26LSL-Luc/+-Smarcb1LoxP/LoxP (R26L/+-SΔ/Δ) E12.5 embryos by trans-uterine injection. H) Histopathological, immunohistochemical, and ultrastructural characterization of tumors generated as described in (G). I) Western blot analysis of tumors generated as described in (G). J-K) Flow cytometry analysis (J) and quantification (K) of protein biosynthesis through OPP (O-propargyl-puromycin) incorporation analysis in tumor-derived cultures generated as described in (G) (n = 4/group). L) Kaplan-Meier survival analysis of mice transplanted orthotopically with Myc proficient (shCtrl) and hypomorphic (shMyc) tumors and assigned to treatment with ixazomib or vehicle (n = 10/group). M) Analysis of apoptotic response by Cleaved Caspase-3 immunostaining in the experimental groups described above. Tumors were harvested for analysis after 10 days of treatment. **** p < 0.0001, NS = not significant by Mantel-Cox test (L) and unpaired two-tailed t-test (E and K). Pooled data are presented as mean ± the standard deviation of biological (E) and technical replicates (K). Size bars: 100 μm unless otherwise specified in panel. See also Figure S4 and Table S2.