Figure 6.
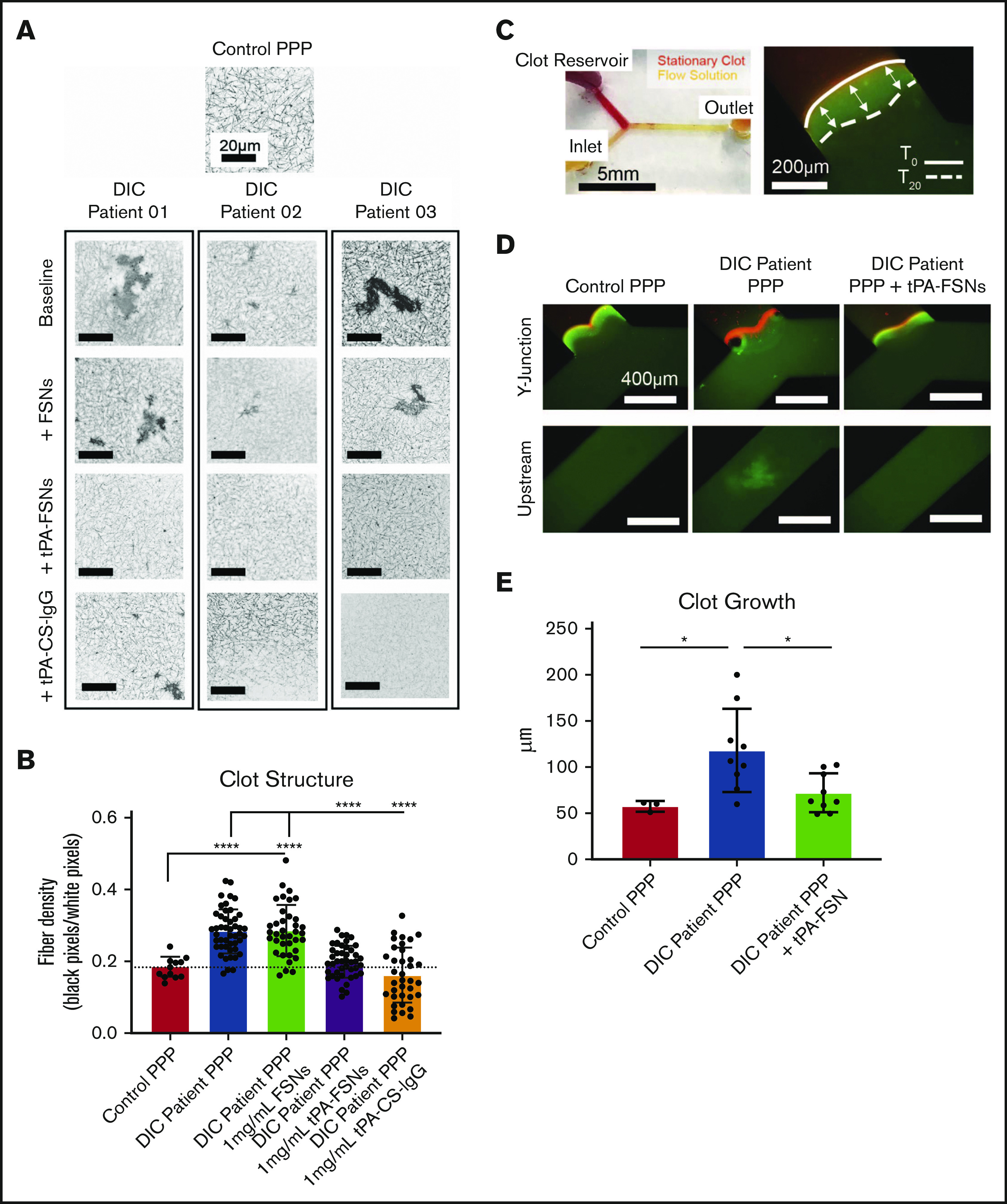
Effects of tPA-FSN treatment on ex vivo DIC patient plasma samples. Confocal microscopy (Zeiss LSM 710 Laser Scanning Microscope) images (A) and fiber density quantification (B) of DIC patient plasma clot samples compared with normal human plasma clots. A C-Apochromat 63× 1.2W objective lens was used to capture 1.89-μm z-stack images, which were analyzed using ImageJ to make 8-bit 3D projections. (B) Fiber density was quantified by determining the ratio of black (fiber) over white (background) pixels in each binary image. Unloaded FSNs, tPA-FSNs, or tPA-CS-IgG (1 mg/mL) were also incorporated into DIC patient plasma clots that were examined with confocal microscopy. Plasma samples from 3 DIC patients were examined, and pooled data from those samples was used for statistical analysis. (C) A custom-made Y-shaped microfluidic device was used to test plasma samples under flow at a wall shear rate of 10 s−1. A stationary fibrin clot was formed at the top Y junction with DIC patient plasma containing Alexa-Fluor 594–labeled fibrinogen for visualization. Flow solutions contained DIC patient plasma and Alexa-Fluor 488–labeled fibrinogen for visualization. (C-D) Images from an EVOS FL Auto Imaging System at 10× magnification were captured at the Y junction and upstream inlet channel and are shown with a reference to how clot growth was measured. (E) Quantification of clot growth at the stationary clot boundary was performed with ImageJ software. Normal healthy human plasma acted as a control. For each plasma sample from a DIC patient (n = 3), 3 trials were conducted with plasma alone in the flow solution, and 3 trials were conducted with plasma containing tPA-FSNs in the flow solution. All data are presented as average ± standard deviation. Data sets were analyzed via a 1-way ANOVA with a Tukey’s post hoc test using a 95% confidence interval. *P < .05; ****P < .0001.
