FIGURE 1.
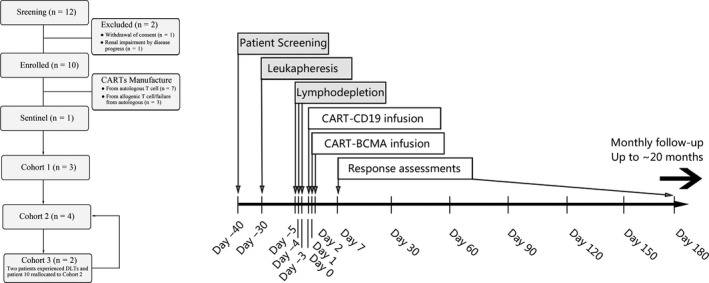
CONSORT diagram and scheme of the clinical trial design. Following patient enrollment, autologous or allogeneic T cells were obtained via leukapheresis and transfected to generate CART‐CD19 or CARTBCMA. After administering short‐term chemotherapy for lymphodepletion (3 doses of cyclophosphamide and fludarabine), patients received one‐dose infusion of CART‐CD19 on day 0 and then a split‐dose infusion of CART‐BCMA over 2 days (40% on day 1 and 60% on day 2). Patients were admitted for 2 weeks for management of potential adverse events, followed by long‐term follow‐up for response assessment. All patients reported here were infused with CAR T cells between July 2017 and November 2017. The presented data cut‐off date was July 31, 2019.
