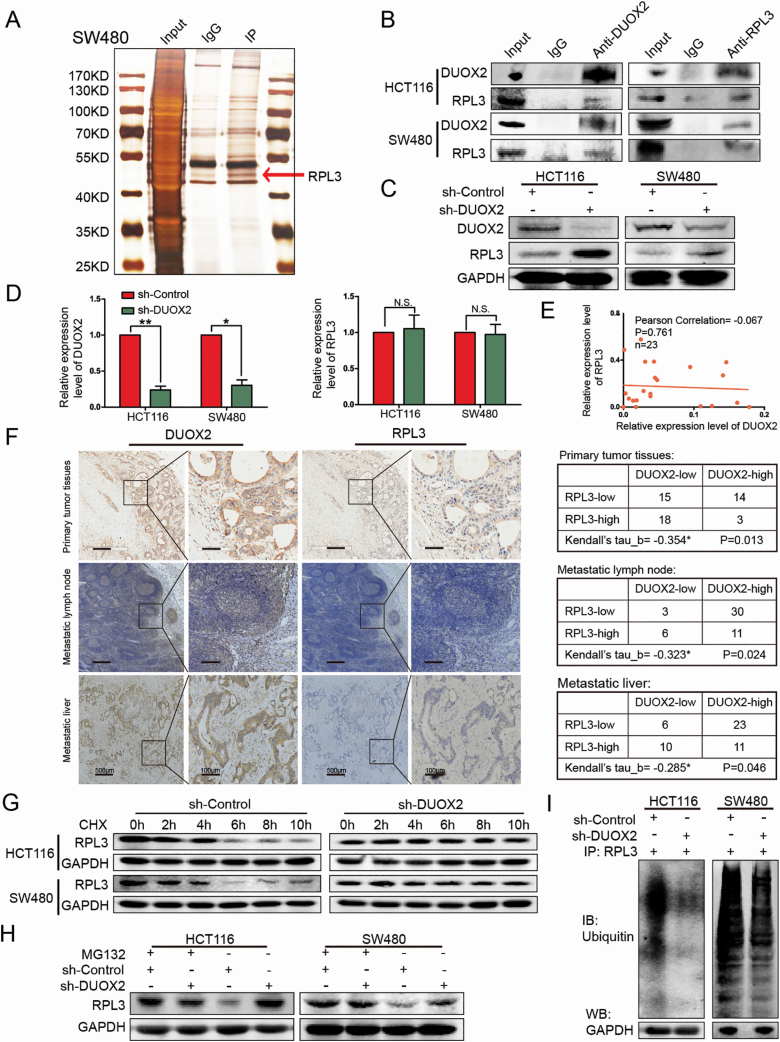
Figure 3.
DUOX2 and RPL3 proteins are negatively correlated, DUOX2 interacts with RPL3 through ubiquitination. (A) The cell lysates were subjected to IP with anti-IgG antibody (IgG) or anti-DUOX2 antibody (IP). Differential bands between anti-DUOX2 and anti-IgG in the silver stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis were detected by MS. (B) The interaction between DUOX2 and RPL3 was proved by means of coimmunoprecipitation. (C) Total protein of HCT116 and SW480 cells, which were knockdown DUOX2 (sh-DUOX2) and the negative control (sh-Control), was extracted and subjected to western blotting using anti-DUOX2, anti-RPL3 and anti-GAPDH antibodies. (D) The expression of RPL3 gene levels after DUOX2 knockdown in HCT116 and SW480 cells. (E) Expression of DUOX2 and RPL3 genes in CRC tissues (n = 23). Statistical analysis was performed using Pearson correlation coefficient. (F) The expressions of DUOX2 and RPL3 protein in different tissues of CRC patients by immunohistochemistry. Kendall’s tau-b (Kendall) rank correlation coefficient was used to reflect the correlation between DUOX2 and RPL3 protein, *P < 0.05. (G) HCT116 and SW480 cells, which were knockdown DUOX2 (sh-DUOX2) and the negative control (sh-Control), were treated with cycloheximide (100 µg/ml), collected at the indicated time points and immunoblotted for RPL3 and GAPDH. (H) HCT116 and SW480 cells, which were knockdown DUOX2 (sh-DUOX2) and the negative control (sh-Control), were treated with MG132 (10 µM) for 12 h, total protein was extracted and subjected to western blotting using anti-RPL3 and anti-GAPDH antibodies. (I) Different groups of cells, sh-Control and sh-DUOX2, were lysed with IP lysis/wash buffer with protease inhibitor and phosphatase inhibitor. Anti-RPL3 antibody was used for IP, and the immune-precipitates were probed with antiubiquitin. The same volume of protein was extracted from each group for western blotting analysis with anti-GAPDH antibody as a loading control. *P < 0.05, **P < 0.01.