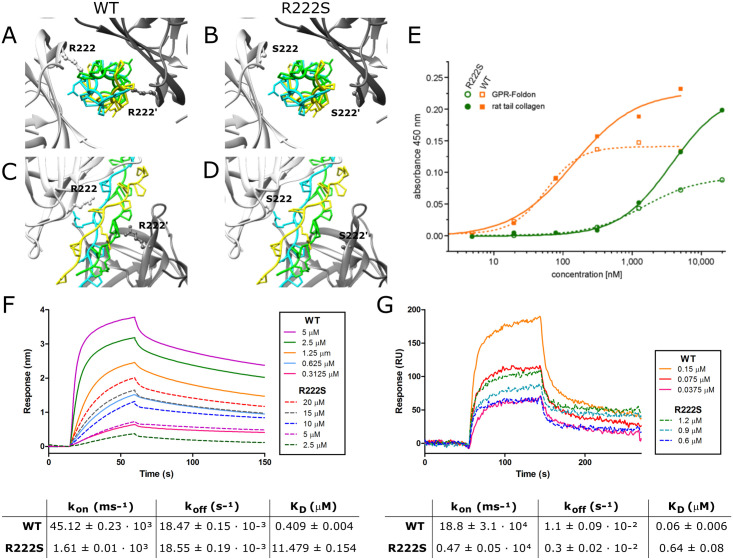
Fig 3. Determination of the binding properties of WT and R222S HSP47.
(A-D) Crystal structure of the HSP47-collagen interaction with the collagen trimer depicted centrally, surrounded by two HSP47 molecules depicted in light and dark grey. (A,C) Wild-type (WT) and (B,D) R222S HSP47. (E) ELISA binding assay of wild-type (WT) and R222S HSP47 to GPR-foldon and type I collagen from rat tail. (F-G) Concentration dependent direct binding of WT and R222S HSP47 proteins with (F) GPR-foldon using biolayer interferometry and (G) type I collagen using surface plasmon resonance. All curves are averaged by a minimum of three measurements. Tables represent the respective binding affinities. Results are shown as mean ± SD.