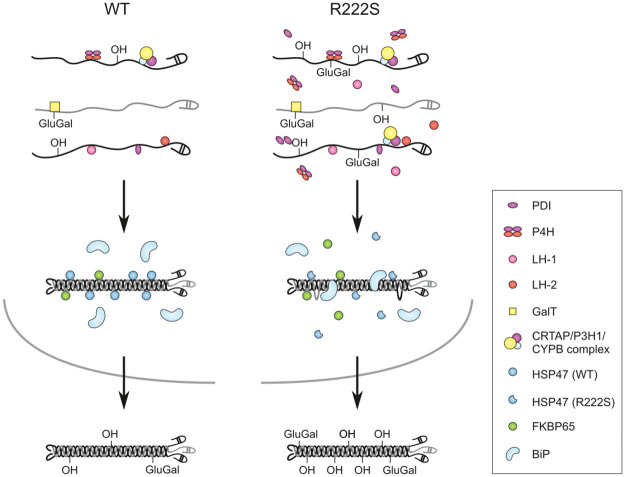
Fig 7. Proposed mechanism of HSP47-R222S.
Wild-type (WT) is depicted on the left, R222S on the right. In R222S fibroblasts, increased levels of modifying enzymes are detected, which can bind the nascent procollagen α1(I)- and α2(I)-chains (depicted in black and grey, respectively) and eventually result in overmodification of the respective pro-α(I)-chains due to an increased enzyme to α-chain ratio. Following trimerisation of the pro-α-chains, HSP47-R222S binds with lower affinity to the procollagen triple helix. The lack of triple helix stabilization by defective HSP47 binding potentially result in local unwinding of the triple helix, thereby making the individual pro-α-chains accessible to the modifying enzymes for additional posttranslational modifications. In addition, the vacant HSP47 binding sites can presumably be occupied by other chaperones (e.g. BiP). This will result in overmodification of pro-α-chains without delaying the folding or secretion of type I procollagen. The overcompensation by the molecular ensemble in combination with the fact that HSP47-R222S can still bind to type I procollagen, allows it to still function as a guide molecule, bind to TANGO1 and direct procollagen molecules to special secretory vesicles, resulting in secretion of overmodified type I procollagen. The incorporation of overmodified type I collagen will subsequently result in aberrant collagen fibrillogenesis.