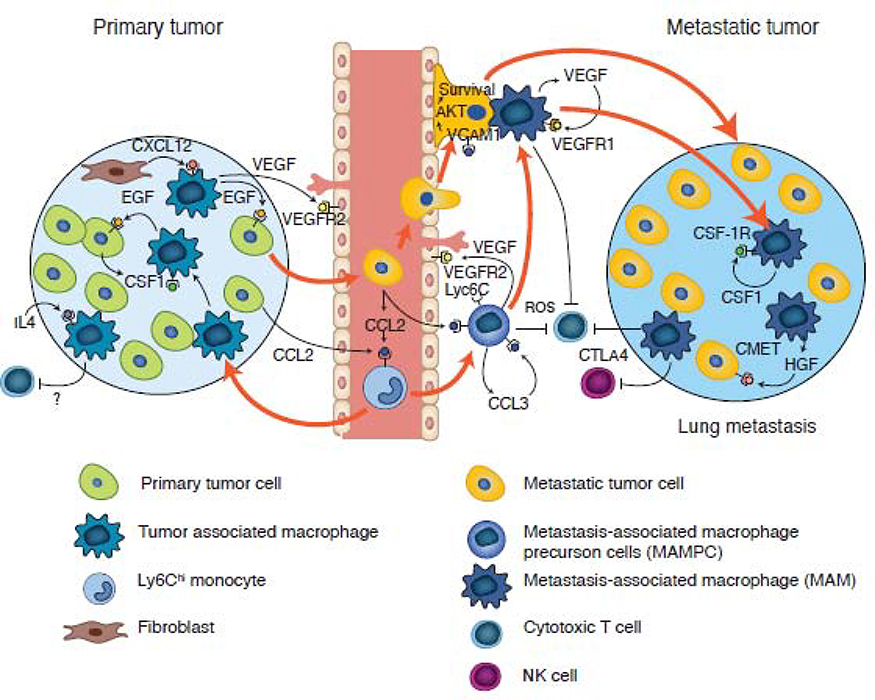
Figure 4. Origin of tumor- and metastasis-associated macrophages in mouse models of breast cancer.
Classical monocytes are recruited to both primary and metastatic sites via a CCL2-CCR2 driven chemokine signaling pathway. In the primary tumor, these recruited monocytes undergo sequential development into TAMs, which are first involved in a streaming behavior that drives tumor cell invasion via a CSF1-Epidermal growth factor (EGF) paracrine loop, before being recruited to vessels by fibroblast expressed CXCL12. On the vessel, these TAMs enhance tumor cell intravasation by expressing VEGFA. At metastatic sites, the circulating tumor cells recruit classical monocytes expressing VEGFA (causing vessel permeability) that upon arrival, promotes the extravasation of the latter. Subsequent signaling pathways (as indicated), result in monocyte differentiation through a progenitor stage (MAMPC) --that is immuno-suppressive via production of reactive oxygen species (ROS)-- into a MAM that confers survival signals to tumor cells via vascular cell adhesion protein 1 (VCAM1) engagement. These MAMs further differentiate, supporting metastatic tumor growth via the depicted signaling pathways; this process occurs in part via inhibition of cytotoxic CD8+ T cells and NK cell effector functions.