Figure 1.
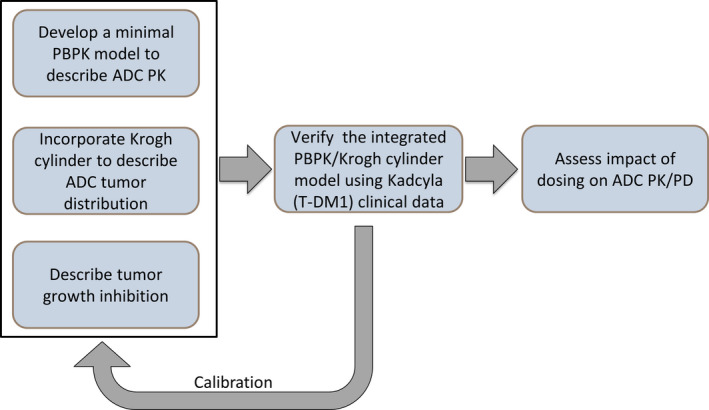
Model development workflow. First, the ADC model was developed by integrating three submodels: (1) a minimal PBPK submodel to capture ADC PK, (2) a Krogh cylinder submodel to capture ADC distribution within the tumor, and (3) a tumor growth inhibition submodel to capture ADC PD. The integrated ADC model was next verified with T‐DM1 clinical PK/PD in metastatic breast cancer as a test case. The verified model was then applied to examine the impact of dosing on PK/PD of a hypothetical ADC. ADC, antibody‐drug conjugate; PBPK, physiologically‐based pharmacokinetic; PD, pharmacodynamic; PK, pharmacokinetic; T‐DM1, Ado‐trastuzumab emtansine.
