Abstract
Background
There is limited data on outcomes in patients with coronavirus disease 2019 (Covid-19) in rural United States (US). This study aimed to describe the demographics, and outcomes of hospitalized Covid-19 patients in rural Southwest Georgia.
Methods
Using electronic medical records, we analyzed data from all hospitalized Covid-19 patients who either died or survived to discharge between 2 March 2020 and 6 May 2020.
Results
Of the 522 patients, 92 died in hospital (17.6%). Median age was 63 years, 58% were females, and 87% African-Americans. Hypertension (79.7%), obesity (66.5%) and diabetes mellitus (42.3%) were the most common comorbidities. Males had higher overall mortality compared to females (23 v 13.8%). Immunosuppression [odds ratio (OR) 3.6; (confidence interval (CI): 1.52–8.47, p=.003)], hypertension (OR 3.36; CI:1.3–8.6, p=.01), age ≥65 years (OR 3.1; CI:1.7–5.6, p<.001) and morbid obesity (OR 2.29; CI:1.11–4.69, p=.02), were independent predictors of in-hospital mortality. Female gender was an independent predictor of decreased in-hospital mortality. Mortality in intubated patients was 67%. Mortality was 8.9% in <50 years, compared to 20% in ≥50 years.
Conclusions
Immunosuppression, hypertension, age ≥ 65 years and morbid obesity were independent predictors of mortality, whereas female gender was protective for mortality in hospitalized Covid-19 patients in rural Southwest Georgia.
KEY MESSAGES
Patients hospitalized with Covid-19 in rural US have higher comorbidity burden.
Immunosuppression, hypertension, age ≥ 65 years and morbid obesity are independent predictors of increased mortality.
Female gender is an independent predictor of reduced mortality.
Keywords: Covid-19, demographics, baseline characteristics, outcomes, rural US
Introduction
The first confirmed case of coronavirus disease 2019 (Covid-19) in the United States (US) was reported from Snohomish County, Washington in January 2020 [1]. Fast forward several weeks, and this novel virus has taken the country and globe by storm. At one point during the outbreak, Albany, Georgia (Dougherty County) ranked fourth in the world for the number of confirmed cases per 100,000 population, only trailing behind New York, New York; Lombardy, Italy; and Wuhan, China [2]. Since the outbreak, this rural and relatively isolated area of Southwest Georgia has been ravaged by the disease.
Dougherty and its surrounding counties have some of sickest patient populations within the state of Georgia. Dougherty County ranks 152nd out of 159 counties in health outcomes in Georgia. The county also ranks number one in the state for the age-adjusted death rate for cardiovascular disease. The surrounding counties follow similar demographics [3,4]. Georgia, as a whole, follows along similar pattern, with the state ranking 40th in the country for overall health outcomes. This includes ranking 38th and 40th for cardiovascular and diabetes related deaths respectively [5].
Since the residents of this region have a higher prevalence of chronic comorbid conditions, it becomes challenging to establish a direct comparison to health outcomes experienced by the general U.S. population with Covid-19. To date, there have been no large studies reporting the outcomes of Covid-19 patients from the rural US. The aims of this study are: (i) to describe the demographics, baseline comorbidities, and outcomes of hospitalized Covid-19 patients in rural Southwest Georgia, (ii) to determine which demographics and comorbidities affected outcomes in these patients.
Materials and methods
This study was conducted at Phoebe Putney Health System, the largest community health system in Southwest Georgia, serving a population of over half a million. The Phoebe Putney institutional review board approved the study and waived the requirement for informed consent due to minimal risk. Patients were admitted to any of the three Phoebe Putney hospitals between 2 March 2020 and 6 May 2020, inclusive of those dates. All hospitalized patients with confirmed Covid-19, who had an outcome, were included in this study. The outcome was defined as either discharge from the hospital (home, nursing home, long term care facility, skilled nursing facility, county jail) or death. Hospitalized patients who did not have an outcome by 6 May 2020 were not included in the study. The patients transferred to another hospital (due to the hospital being at full capacity or need for treatment not available at our facility) were not included as well.
Data were collected from the electronic medical records (Meditech and Athena Health). All patients were confirmed positive for Covid-19 by nasopharyngeal swab using a polymerase chain reaction (PCR) test. Patients were considered to have confirmed infection if the initial test result was positive, or if it was negative but repeat testing was positive. Repeat tests were performed on inpatients during hospitalization shortly after initial test results were available if there was a high clinical pre-test probability of Covid-19, or if the initial negative test result had been judged likely to be a false-negative due to poor sample collection. Transfers from one in-system hospital to another were merged and considered as a single visit.
Data collection included demographics, insurance, baseline comorbidities, tobacco use, alcohol use, illicit drug use, home medications, symptoms on presentation, vitals, initial laboratory tests, initial electrocardiogram, the severity of presenting illness, treatment course (intensive care unit (ICU) care, need for mechanical ventilation and dialysis), length of stay (LOS) and outcomes (discharged alive or death). The comorbidities included hypertension (HTN), diabetes mellitus (DM), coronary artery disease (CAD), congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), asthma, chronic kidney disease (CKD), cancer, immunosuppression and chronic liver disease. All comorbidities except immunosuppression were adjudicated based on the 10th version of the International Classification of Diseases (ICD-10). Patients were considered immunosuppressed if they had been on chronic steroids or other immunosuppressive therapy. To calculate LOS, we used the actual admission and discharge times. The total LOS in hours was divided by 24 to get the LOS in days.
We utilized Statistical Analysis Software (SAS) version 9.4 for all the statistical analyses. Categorical variables were expressed in numbers and percentages and analyzed using Pearson’s Chi Square test. For continuous variables such as age and LOS, we used median instead of mean since the data was not normally distributed. Age and body mass index (BMI) were divided into categories, and considered categorical variables for multivariate analysis. Hierarchical multivariable logistic regression analysis was used to identify the independent predictors of in-hospital mortality, need for intubation and need for new dialysis. We included age, BMI, gender, race and all the baseline comorbidities in this model. C-statistics was above 0.7 for all the models to be acceptable.
All the authors reviewed the manuscript and take responsibility for the accuracy and completeness of the data presented here.
Results
A total of 522 patients were included in this analysis. The median age was 63 years (interquartile range {IQR}, 50–72 years) with 58.2% females (Table 1). A total of 507 patients (97.1%) had an initial positive test, and 15 patients (2.9%) had a positive test on repeat testing. African Americans comprised the majority of patients (87%). The most common comorbidities were HTN (n = 416, 79.7%), obesity (n = 347, 66.5%) and DM (n = 221, 42.3%). Morbid obesity was present in 25.6% of patients (Table 1). The majority of the patients were insured with 54.2% Medicare beneficiaries, and 29.7% with commercial insurance. Only 11.1% of the patients were uninsured (Table 1).
Table 1.
Demographics and comorbidities in hospitalized Covid-19 patients.
| Demographics | Total population–N(%)=522 (100) | Discharged–N(%)=430 (82.4%)a | Died–N(%)=92 (17.6%)b |
|---|---|---|---|
| Age (median, IQR) (yrs) | 63 (50−72) | 60 (50−70) | 70 (1−78) |
| Femalec | 304 (58.2) | 262 (60.9) | 42 (45.7) |
| African American | 454 (87) | 375 (87.2) | 79 (86) |
| Caucasian | 59 (11.3) | 46 (10.7) | 13 (14.1) |
| Commercial | 155 (29.7) | 143 (33.3) | 12 (13) |
| Medicare | 283 (54.2) | 210 (48.8) | 73 (79.3) |
| Medicaid | 25 (4.8) | 20 (4.7) | 5 (5.4) |
| Uninsured | 58 (11.1) | 57 (13.3) | 1 (1.1) |
| Other (Tricare) | 1 (0.2) | 0 (0) | 1 (1.1) |
| Comorbidities/risk factors | |||
| Hypertension | 416 (79.7) | 330 (76.7) | 86 (93.8) |
| Diabetes mellitus | 221 (42.3) | 169 (39.3) | 52 (56.5) |
| Coronary artery disease | 48 (9.2) | 35 (8.1) | 13 (14.1) |
| Congestive heart failure | 70 (13.4) | 50 (11.6) | 20 (21.7) |
| Chronic obstructive pulmonary disease | 47 (9) | 34 (7.9) | 13 (14.1) |
| Asthma | 68 (13) | 57 (13.2) | 11 (12) |
| Chronic kidney disease | 78 (14.9) | 58 (13.5) | 20 (21.7) |
| End stage renal disease | 30 (5.8) | 23 (5.3) | 7 (7.6) |
| Obesity (BMI ≥ 30) | 347 (66.5) | 290 (67.4) | 52 (62) |
| Morbid obesity (BMI ≥ 40) | 134 (25.6) | 111 (25.8) | 23 (25) |
| Cancer | 48 (9.2) | 39 (9.1) | 9 (9.8) |
| Immunosuppression | 29 (5.6) | 17 (4) | 12 (13) |
| Chronic liver disease | 6 (1.1) | 4 (0.9) | 2 (2.1) |
| Former/current smoker | 89 (17) | 70 (16.3) | 18 (20.7) |
IQR: interquartile range; BMI: body mass index.
aPercentage based on denominator N = 522, remaining percentages in this column are based on denominator N = 430.
bPercentage based on denominator N = 522, remaining percentages in this column are based on denominator N = 92.
cOverall mortality in females was 13.8% and in males was 23%.
Out of the 522 patients, 92 died (17.6%), and 430 were discharged alive (82.4%). The median age was higher in those who died compared to those discharged alive (70 v 60 years). Males had higher mortality compared to females (23 v 13.8%). Of those who were discharged, 87% were discharged home. Remaining patients were discharged either to a skilled nursing facility, nursing home, rehabilitation facility, short/long term acute care facility.
Median LOS was 6 days (IQR, 4–11 days). A total of 146 patients (28%) had pneumonia, and 269 patients (51.5%) had severe pneumonia on admission (Table 2). Intensive care unit (ICU) care was required in 123 patients (23.6%), whereas 97 patients (18.6%) were intubated during the hospital stay. A total of 30 patients (5.8%) needed dialysis (they were not on dialysis prior to admission) during the hospital stay (Table 2).
Table 2.
Length of stay, severity of presenting symptoms, intubation, renal replacement and mortality in hospitalized Covid-19 patients.
| Total population–N(%)=522 | Discharged–N(%)=430 (82.4%)a | Died–N(%)=92 (17.6%)b | |
|---|---|---|---|
| LOS (Median, IQR) (days) | 6 (4−11) | 6 (3−10) | 10 (6−16) |
| Asymptomatic | 12 (2.3) | 12 (2.8) | 0 (0) |
| Mild symptoms | 78 (14.9) | 69 (16) | 9 (9.8) |
| Pneumonia | 146 (28) | 140 (32.6) | 6 (6.5) |
| Severe pneumoniac | 269 (51.5) | 198 (46) | 71 (77.2) |
| Septic shockc | 4 (0.8) | 2 (0.5) | 2 (2.2) |
| ARDSc | 8 (1.5) | 6 (1.4) | 2 (2.2) |
| ICU care (anytime during hospitalization) | 123) (23.6) | 52 (12/1) | 71 (77.2) |
| Intubation (anytime during hospitalization)d | 97 (18.6) | 32 (7.4) | 65 (70.7) |
| Need for new renal replacement therapy (during hospitalization) | 30 (5.8) | 8 (1.9) | 22 (23.9) |
LOS: length of stay; IQR: interquartile range; ARDS: Acute respiratory distress syndrome; ICU: intensive care unit.
aPercentage based on denominator N = 522, remaining percentages in this column are based on denominator N = 430.
bPercentage based on denominator N = 522, remaining percentages in this column are based on denominator N = 92.
cThe numbers represent these findings on presentation.
dMortality in intubated patients was 67% (65/97).
The median LOS was higher in those who died (10 days; IQR, 6–16 days) compared to those discharged alive (6 days; IQR, 3–10 days). Those who died presented more often with severe pneumonia compared to those discharged alive (77.2 v 46%). The need for ICU care (77.2 v 12.1%), intubation (70.7 v 7.4%), and new renal replacement therapy (23.9 v 1.9%) anytime during hospitalization was higher in the patients who died compared to those discharged alive (Table 2). The overall mortality in intubated patients was 67%.
Based on multivariable logistic regression analysis, immunosuppression [odds ratio (OR) 3.6; (confidence interval (CI): 1.52–8.47, p=.003)], HTN (OR 3.36; CI:1.3–8.6, p=.01), age ≥65 years (OR 3.1; CI:1.7–5.6, p<.001) and morbid obesity (OR 2.29; CI:1.11–4.69, p=.02), were independent predictors of in-hospital mortality. Diabetes Mellitus and CHF showed trend towards increased mortality but were not statistically significant. Female gender was an independent predictor of decreased mortality (OR 0.41; CI:0.24–0.70, p=.001) (Table 3). Obesity, immunosuppression and age ≥65 years were independent predictors for intubation during hospitalization. Morbid obesity, DM and age ≥65 years were independent predictors of need for new renal replacement therapy during hospitalization (data not shown). Patients with higher burden of independent predictors had significantly higher mortality. The mortality was 1.9% with 0, 10.2% with 1, 21.7% with 2, 40.5% with 3, and 66.7% with 4, cumulative independent predictors (Figure 1).
Table 3.
Predictors of in-hospital mortality in Covid-19 positive patients.
| Variable | Odds ratio (confidence interval) | p Value |
|---|---|---|
| Age ≥65 years | 3.1 (1.7–5.6) | <.001 |
| BMI groups | ||
| < =30 | Reference | |
| 30–40 | 1.49 (0.79–2.77) | .21 |
| > =40 | 2.29 (1.11–4.69) | .02 |
| Female vs. male | 0.41 (0.24–0.70) | .001 |
| Black vs. White | 0.82 (0.37–1.78) | .61 |
| Comorbidities | ||
| Hypertension | 3.36 (1.3–8.6) | .01 |
| Coronary artery disease | 1.17 (0.54–2.5) | .68 |
| Congestive heart failure | 1.58 (0.81–3.1) | .17 |
| Chronic obstructive pulmonary disease | 1.48 (0.65–3.34) | .34 |
| Asthma | 0.74 (0.33–1.64) | .46 |
| Chronic kidney disease | 1.08 (0.51–2.28) | .82 |
| Diabetes mellitus | 1.51 (0.9–2.55) | .11 |
| Immunosuppression | 3.6 (1.52–8.47) | .003 |
| Chronic liver disease | 1.89 (0.23–15.44) | .55 |
| Cancer | 0.48 (0.2–1.1) | .09 |
| Tobacco smoking | 1.03 (0.55–1.93) | .91 |
Figure 1.
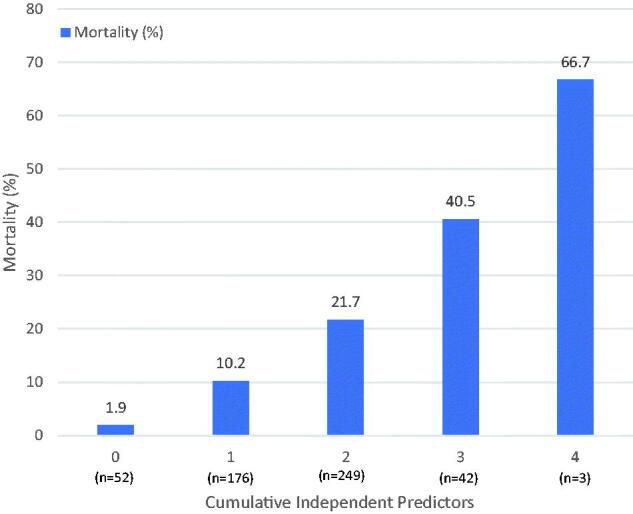
Histogram showing increasing mortality with increased cumulative burden of independent predictors. Independent predictors include–age ≥ 65 years, hypertension, immunosuppression, and morbid obesity.
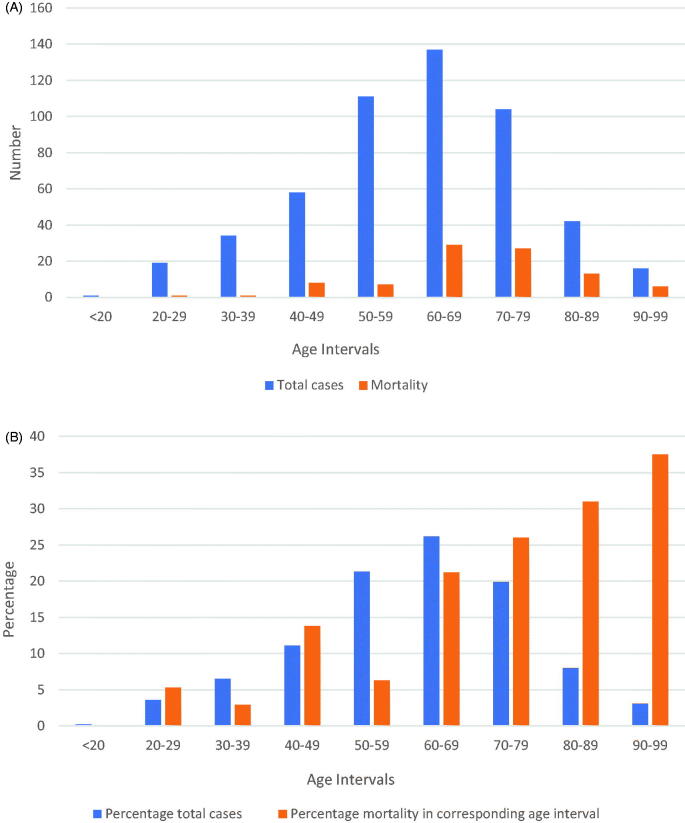
There was only one patient <20 years of age (aged 19 years). A total of 21.5% patients were <50 years old. The mortality in <50 years old was 8.9%, whereas the mortality in >50 years was 20%. One patient died each in age groups 20–29 and 30–39 years, with a mortality rate of 5.3% and 2.9% respectively. Mortality rate in the age group 40–49 years was 13.8%. Mortality increased with every 10-year age intervals. Table 4 provides discharge disposition by 10-year age intervals. There were 16 patients in the age group 90–99 years and mortality rate was 37.5%. The need for ICU care (28.8%) and intubation (23.1%) was highest in the 70- to 79-year age group. Figure 2, panel A shows a histogram of the total cases and mortality by 10-year age intervals. Figure 2, panel B shows a histogram of the percentage of total cases and percentage mortality (in that 10-year interval) by 10-year age intervals.
Table 4.
Discharge disposition by 10-year age interval in hospitalized Covid-19 patients.
| Age interval, yrs | Discharged alive, No./No. (%) |
Died, No./No. (%) |
Total mortality, No./No. (%) | Need for ICU care (all patients) No./No. (%) | Intubation (all patients) No./No. (%) | ||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | ||||
| <20 | n/a | 1/1 (100) | n/a | n/a | 0 | 0/1 | 0/1 |
| 20–29 | 7/7 (100) | 11/12 (91.7) | 0/7 (0) | 1/12 (8.3) | 1/19 (5.3) | 1/19 (5.3) | 1/19 (5.3) |
| 30–39 | 13/14 (92.9) | 20/20 (100) | 1/14 (7.1) | 0/20 (0) | 1/34 (2.9) | 6/34 (17.6) | 5/34 (14.7) |
| 40–49 | 24/28 (85.7) | 26/30 (86.7) | 4/28 (14.3) | 4/30 (13.3) | 8/58 (13.8) | 17/58 (29.3) | 10/58 (17.2) |
| 50–59 | 37/42 (88.1) | 67/69 (97.1) | 5/42 (11.9) | 2/69 (2.9) | 7/111 (6.3) | 23/111 (20.7) | 19/111 (17.1) |
| 60–69 | 37/50 (74) | 71/87 (81.6) | 13/50 (26) | 16/87 (18.4) | 29/137 (21.2) | 35/137 (25.5) | 31/137 (22.6) |
| 70–79 | 34/54 (63) | 43/50 (86) | 20/54 (37) | 7/50 (14) | 27/104 (26) | 30/104 (28.8) | 24/104 (23.1) |
| 80–89 | 14/19 (73.7) | 15/23 (65.2) | 5/19 (26.3) | 8/23 (34.8) | 13/42 (31) | 9/42 (21.4) | 6/42 (14.3) |
| 90–99 | 2/4 (50) | 8/12 (66.7) | 2/4 (50) | 4/12 (50) | 6/16 (37.5) | 2/16 (12.5) | 1/16 (6.3) |
Figure 2.
Panel A–histogram showing total cases and mortality by 10-year age interval, Panel B–histogram showing percentage of total cases and percentage mortality in corresponding age interval by 10-year age intervals.
Discussion
To our knowledge, this is the first study to describe the demographics, outcomes and independent predictors of mortality in Covid-19 patients in the rural US population. The overall mortality in our study was 17.6%. This is slightly lower compared to the largest case series from New York (NY), despite much higher risk factor burden in our population, including HTN (79.7 v 56%), DM (42.3 v 34%), CKD (14.9 v 5%), CHF (13.4 v 6.9%), COPD (9 v 5.4%) and obesity (66.5 v 41.7%) [6]. Compared to the population in NY, higher proportion of our patients needed ICU care (23.6 v 14.2%) and mechanical ventilation (18.6 v 12.2%). Our mortality in intubated patients was also lower than NY (67 v 88%) [6]. This study from NY did not report outcomes on more than 50% patients, while we had outcomes available for all our patients [6]. This can possibly explain the different findings in our study. The findings from our study cannot be compared to the study from Wuhan, China, reporting a mortality of 1.4%. In that study, 1029 of the 1099 patients (93.6%) were still hospitalized by the time of study cut-off date [7]. Also, the population was younger (median age of 47 years), and had far fewer risk factors (HTN − 15%, DM − 7.4%, CAD − 2.5%, COPD − 1.1%) [7]. Our mortality in patients needing ICU care was 58%, lower than a small study from Washington state, which reported 67% mortality amongst 21 ICU patients [8]. A large case series from Italy reported outcomes on 1591 hospitalized Covid-19 patients in the ICU [9]. The ICU mortality was 26% by the time of study cut-off date. Their data cannot be compared to ours, since 58% patients were still in the ICU at the time of study completion, and only 16% were discharged from the ICU [9]. Their underlying risk factor burden was also lower compared to our study (HTN − 49%, DM − 17%, COPD − 4%, CKD − 3%) [9].
Cardiovascular disease (CAD and/or CHF) in our population was 22.6%, comparable to ICU only population in Italy at 21%. Smoking was also more prevalent in the Albany population at 17% of all admitted patients, compared to 14.5% in China and 15.6% in New York City. Asthma rate was also higher in Albany at 13%, compared to 9% in New York City and 9.1% in Washington [6–9]. Our study had much higher proportion of females (58 v 42% in China, 39% in NY and 18% in Italy) [6,7,9]. African-Americans consisted of the majority of our study population, which might be another reason our findings may not be directly comparable to other places in the US. Age ≥65 years was independent predictor of in-hospital mortality in our study, a finding consistent with prior studies [10,11]. Other than age ≥65 years, HTN, immunosuppression and morbid obesity were independent predictors of in-hospital mortality in our study. Contradictory to some studies, we did not find CAD and CHF as independent predictors of in-hospital mortality, although CHF (as well as DM) showed trend towards increased mortality [10,12]. This could be attributed to our demographically distinct population with significantly higher proportion of females compared to other studies. Our findings also suggest female gender to be an independent predictor for decreased mortality. Also, the meta-analysis by Aggarwal et al., reported the association of combined cardiovascular disease (defined as any cardiac pathology except HTN) and Covid-19 mortality [12]. Their overall sample size was much higher than ours (n = 4858 vs. n = 522) [12]. We did find trend for increased mortality with CHF in our study. With bigger sample size and combining CAD and CHF as a predictor, we might be able to show cardiovascular disease as an independent predictor of mortality.
This study outlines that the population infected with SARS-CoV-2 in Southwest Georgia is demographically distinct, with higher comorbidities burden, compared to other hotspot areas around the world including multiple provinces in China, Lombardy, Italy and New York City.
Our study has certain limitations that need to be acknowledged. First, at the time of study completion, there were 78 active Covid-19 patients in the hospital. Second, our study did not include the data on 96 positive patients who were transferred to other facilities (mainly due to lack of beds at our facility) during the study period. Third, since the follow up time was short, the true mortality of this disease cannot be estimated as some patients may die later from complications of the disease or prolonged hospitalization itself. Finally, this was a retrospective study with data abstraction from the electronic medical record, and hence some data elements might not be accurately captured.
Conclusions
This study describes the demographics, comorbidities and outcomes of hospitalized Covid-19 patients in rural Southwest Georgia, with higher baseline comorbidities than general US population. The overall mortality was 17.6%, with higher mortality in males compared to females. The mortality in intubated patients was 67%. Immunosuppression, HTN, age ≥65 years and morbid obesity were independent predictors of in-hospital mortality, whereas female gender was protective against in-hospital mortality.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Holshue ML, DeBolt C, Lindquist S, Washington State 2019-nCoV case investigation team, et al.. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katz J, Katz MS. Coronavirus deaths by U.S. state and country over time: daily tracker. The New York Times. Available from: https://nyti.ms/2UcjlBD.
- 3.Georgia Department of Public Health. Online analytical statistical information system . Georgia Department of Public Health. Available from: http://oasis.state.ga.us.
- 4.University of Wisconsin Population Health Institute. County health rankings state report 2020. Available from: https://www.countyhealthrankings.org.
- 5.United Health Foundation . 2019. Georgia summary 2019. America’s health rankings. United Health Foundation. Available from: Americashealthrankings.org.
- 6.Richardson S, Hirsch JS, Narasimhan M, and the Northwell COVID-19 Research Consortium, et al.. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323(20):2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W, Ni Z, Hu Y, China Medical Treatment Expert Group for Covid-19, et al.. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arentz M, Yim E, Klaff L, et al. . Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grasselli G, Zangrillo A, Zanella A, for the COVID-19 Lombardy ICU Network, et al.. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region Italy. JAMA. 2020;323(16):1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du R, Liang L, Yang C, et al. . Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5):2000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aggarwal G, Cheruiyot I, Aggarwal S, et al. . Association of cardiovascular disease with coronavirus disease 2019 (COVID-19) severity: a meta-analysis. Curr Probl Cardiol. 2020;45(8):100617. [DOI] [PMC free article] [PubMed] [Google Scholar]