Figure 1: Mounting and hydrating embryos prior to dissection.
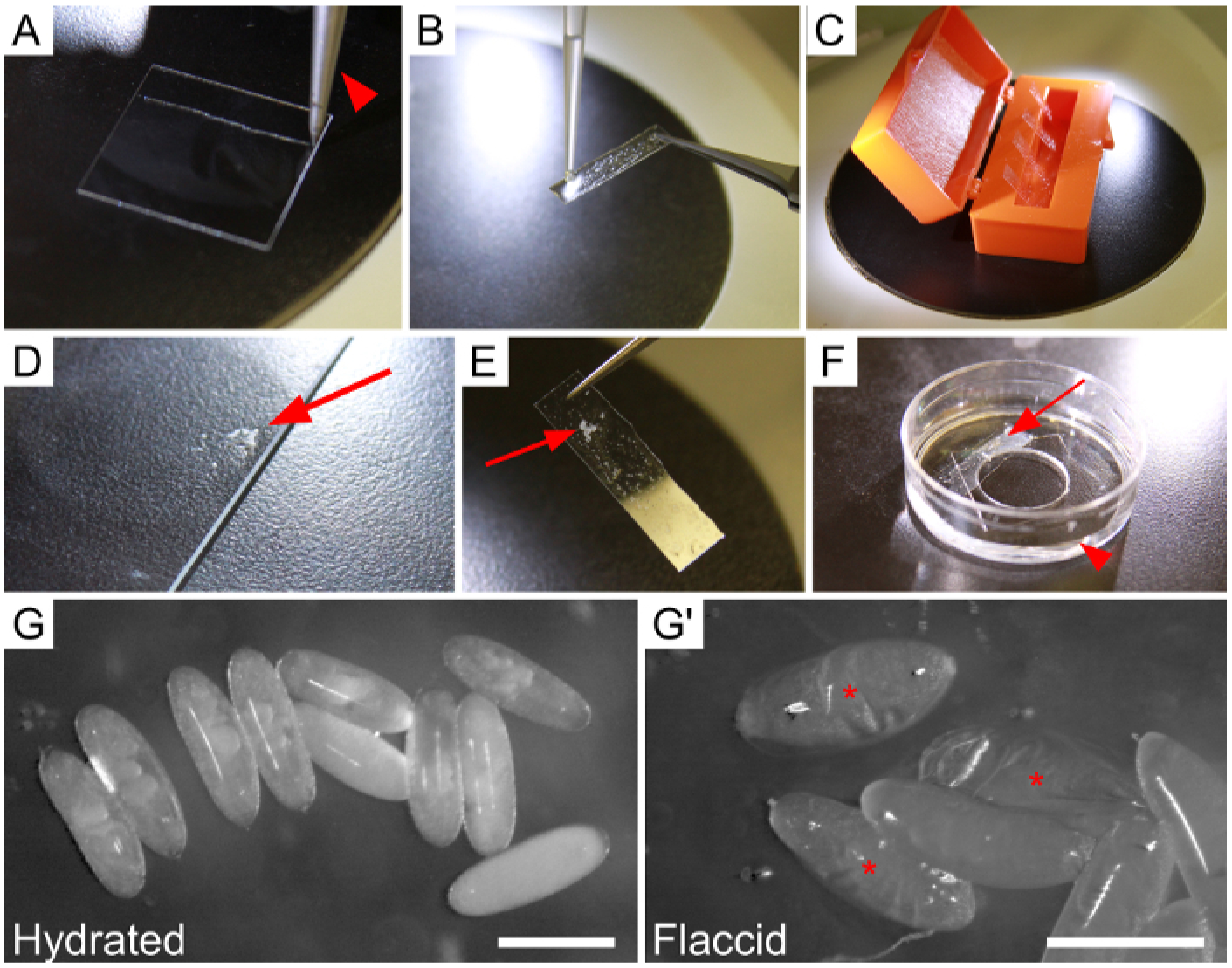
(A–F) Steps for adhering embryos to a strip of glue-coated coverslip, and securing the strip to an imaging dish. (A) Coverslip scored once by diamond-tipped knife (knife indicated by arrowhead) to create a strip. (B) Application of heptane-glue to severed coverslip strip, held with a pair of forceps. (C) Four glue-coated strips drying in a coverslip box. (D–E) Arrows point to embryos. (D) Dechorionated embryos that have been collected in heptane and expelled onto the edge of a microscope slide. Excess heptane was removed with a kimwipe. (E) Glue-coated strip with embryos attached. (F) The final setup of the dish immediately prior to dissection. Note the shallow layer of Ringer’s solution (arrowhead) and placement of the strip (arrow) above the inner dissection circle. (G–G’) Embryos adhered to the strip in the dish. (G) Properly-hydrated, turgid embryos. (G’) Embryos that have become dehydrated due to prolonged air exposure, evident by collapsed vitelline membranes. Asterisks indicate flaccid embryos. Scale bars are 0.5 mm.
