Description
A 12-year-old Caucasian girl was referenced to a paediatric endocrinology consultation for extensive acanthosis nigricans (AN), hirsutism and severe obesity (Body Mass Index (BMI): 37.5 kg/m2, z-score 3.57). She had a normal psychomotor development. Her mother and her maternal grandfather had obesity. Examination revealed Tanner 5 with deep voice, hirsutism (Ferriman and Gallwey score 14), abdominal and lumbar striae, and severe lesions of AN involving the neck, armpits, intermammary cleft and inframammary area (Burke score1 11/14) (figure 1). Biochemical evaluation revealed dyslipidaemia (triglycerides 213 mg/dL), insulin resistance (IR) (insulin 43.5 μUI/mL, HOMA-IR 10.2), high testosterone (74 ng/dL) and low sex hormone-binding globulin (8 nmol/L). She had menarche and there was no oligomenorrhea. Congenital adrenal hyperplasia and hypercortisolism were excluded. Oral glucose tolerance test was normal. In order to screen for a virilising tumour, an abdominal MRI was performed, showing regular adrenal glands but large ovaries (right ovary 27 mm and left ovary 29 mm) with multiple millimetric cysts related to polycystic ovary syndrome (POS). Treatment comprised dietary and physical activity counselling, as well as a gradual treatment with metformin, pioglitazone, spironolactone and oral combined hormonal contraceptive. After 3 years of follow-up, although maintaining a BMI z-score of 3.57, there was partial regression of AN (figure 2) and improvement in the biochemical evaluation: absence of dyslipidaemia and lower testosterone (50.84 ng/dL). IR has worsened (insulin 51.9 μUI/mL, HOMA-IR 12.4), which may be due to the absence of weight loss and to pubertal physiological IR.
Figure 1.
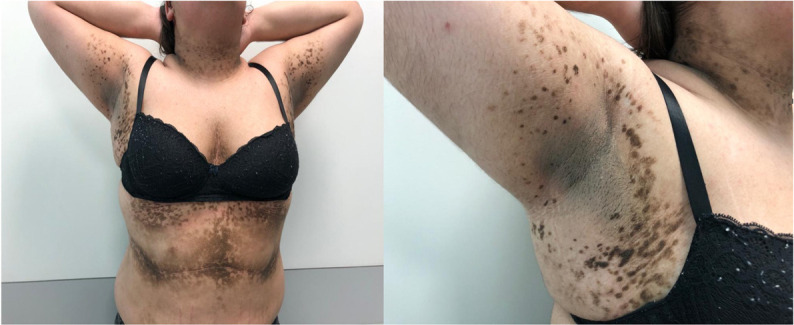
Severe acanthosis nigricans involving neck, armpits, intermammary cleft and inframammary area and abdominal striae; recorded at first consultation, aged 12 years.
Figure 2.
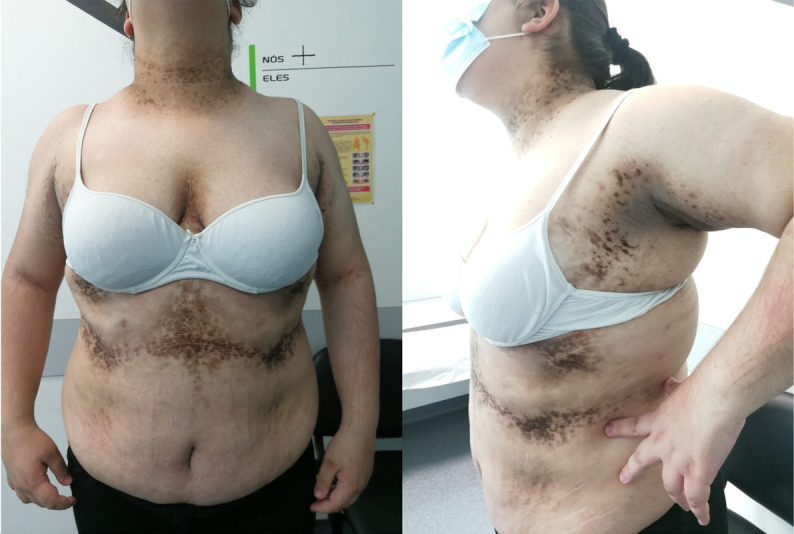
Lighter skin lesions of acanthosis nigricans especially on the neck, armpits and intermammary cleft; aged 15 years.
AN is characterised by dark and thickened skin, symmetrically distributed more frequently on the neck, axillae and groin folds. AN is usually associated with obesity, POS, type 2 diabetes, monogenic causes of IR and malignancy (particularly, gastric carcinoma, Wilm’s tumour and virilising tumours). Paraneoplastic syndromes are often associated with severe AN.2
In AN related to obesity, increased insulin activates keratinocyte insulin-like growth factor (IGF) receptors, particularly IGF-1. At high concentrations, insulin may displace IGF-1 from IGF-binding proteins. Increased circulating IGF leads to keratinocyte and dermal fibroblast proliferation. Among patients with POS, AN is associated with higher free testosterone levels, which may be explained by the association with hyperinsulinaemia, which can promote ovarian thecal androgen secretion and inhibit hepatic synthesis of sex hormone-binding globulin, as in the case of our patient.3
AN is a manageable condition, however, the complete reversion of the lesions is difficult to achieve. Although weight reduction is acknowledged as the most efficient strategy to tackle AN, this report suggests that metformin and pioglitazone should be considered in order to improve insulin sensitivity and endocrine and metabolic indices, which may have a visible impact on AN extension.3 4
In this case, despite the lack of hyperandrogenic signs (acne, alopecia or oligomenorrhea), it was imperative to exclude adrenal and abdominal malignancies. This report highlights a rare case of severe AN in which the aetiology was a non-malignant pathology: C phenotype POS.
Learning points.
Severe acanthosis nigricans (AN) is rare and may be associated with malignancy or syndromic conditions.
In case of patients with polycystic ovary syndrome (POS), AN is associated with higher free testosterone levels which are related to a hyperinsulinaemic state.
Improvement of insulin sensitivity and free testosterone levels with metformin and pioglitazone may be determinant to manage AN in patients with POS.
Footnotes
Contributors: CM conceptualised and wrote the article. MRS, MMG and AA offered guidance and suggestions, and edited the article. All authors participated in patient care and approved the final version of the article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Videira-Silva A, Albuquerque C, Fonseca H. Acanthosis nigricans as a clinical marker of insulin resistance among overweight adolescents. Ann Pediatr Endocrinol Metab 2019;24:99–103. 10.6065/apem.2019.24.2.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das A, Misra P, Panda S. Childhood acanthosis nigricans. Indian J Paediatr Dermatol 2019;20:199–204. 10.4103/ijpd.IJPD_34_18 [DOI] [Google Scholar]
- 3.Das A, Datta D, Kassir M. Acanthosis nigricans: a review. J Cosmet Dermatol 1865;2020:1857. [DOI] [PubMed] [Google Scholar]
- 4.Trent M, Gordon CM. Diagnosis and management of polycystic ovary syndrome in adolescents. Pediatrics 2020;145:S210–8. 10.1542/peds.2019-2056J [DOI] [PubMed] [Google Scholar]


