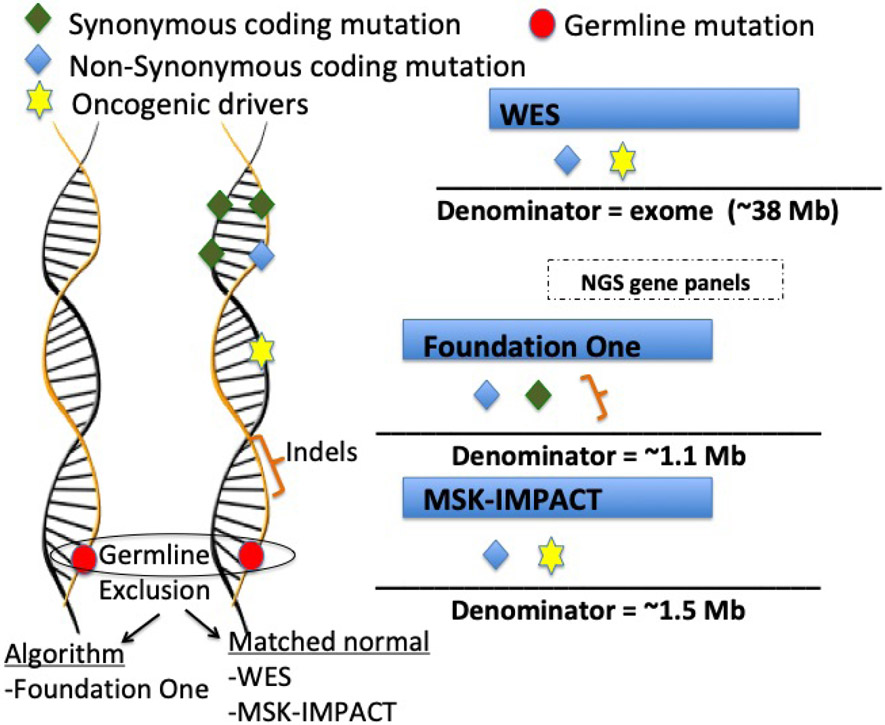
Figure 1 – Schematic representation of the main FDA-approved assays for TMB estimation as well as whole exome sequencing calculation.
Two of the NGS gene panels are FDA-approved tests (Foundation One (Frampton et al., 2013) and MSK-Impact) (Cheng et al., 2015). Symbols represent genetic alterations that are captured as mutations, while the denominator refers to the genome region that is considered for each test. The Foundation Medicine TMB assay examines a genomic region of approximately 1.1 Mb. For TMB estimation this test includes synonymous and non-synonymous mutations and short indels, while oncogenic drivers are excluded. In addition, germline alterations are excluded based on validated bioinformatics algorithms. The MSK IMPACT TMB assay examines approximately 1.5 Mb and, similar to WES, includes non-synonymous mutations in coding regions and oncogenic drivers. Germline alterations are excluded by subtracting matched normal samples.
Examples of other commercial available assays include: Illumina TruSight 500 (~2Mb exome coverage [i.e region sequenced]), Thermo Fisher Scientific Oncomine (1.7 Mb exome coverage), Caris Molecular Intelligence (1.7 Mb exome coverage); NEO New Oncology NEOplus v2 RUO (1.1 Mb exome coverage); TruSight Tumor 170 (0.5 Mb exome coverage); Tempus Plataform (2.4 Mb exome cover)
Abbreviations: Mb = megabase; WES: Whole exome sequencing