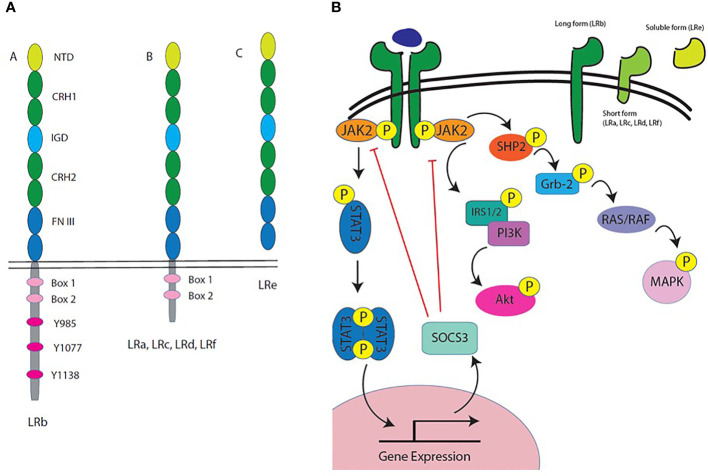
Figure 1.
Leptin receptor isoforms and intracellular signaling. (A) Leptin receptor is composed of an extracellular domain, a transmembrane domain, and a cytoplasmic domain. All variants of the leptin receptor include the extracellular domain. The extracellular domain is composed of several protein motifs: the N terminal domain (NTD), two cytokine receptor homology (CRH) domains that make up the leptin binding site, an immunoglobulin-like domain (IGD), and two fibronectin type 3 (FN III) domains. The cytoplasmic domain of leptin receptor varies between isoforms. LRb, the long form receptor, includes two box domains and several tyrosine residues important for leptin receptor signaling. The other leptin receptor variants are labeled LRa, LRc, LRd, LRf and they all have the complete extracellular binding domain, but their intracellular tails differ; however, they all contain the two box domains. There is also a soluble form of leptin receptor in both humans and mice called LRe. In mice, LRe is directly secreted, while in humans, LRe is generated by ectodomain shedding (metalloproteases cut the receptor off the surface). (B) Leptin receptor isoforms are generated by alternative splicing or processing at the cell membrane. The long form of leptin receptor, also known as LRb, is the only known receptor variant that is capable of signaling through the JAK-STAT pathway. LRb has a long intracellular tail that includes several tyrosine residues that are phosphorylated for signal transduction by JAK2. LRb signaling primarily occurs through the JAK2/STAT3 pathway, with STAT3 translocating to the nucleus to modify gene expression. LRb also signals through the PI3K/Akt pathway and the MAPK pathway. These pathways in immune cells have been shown to lead to metabolic and functional changes, which could account for the pleiotropic effects of leptin on different immune cell types (56–58).