Figure 1. Identification of Cdk1 phosphorylation sites in Net1 in vitro.
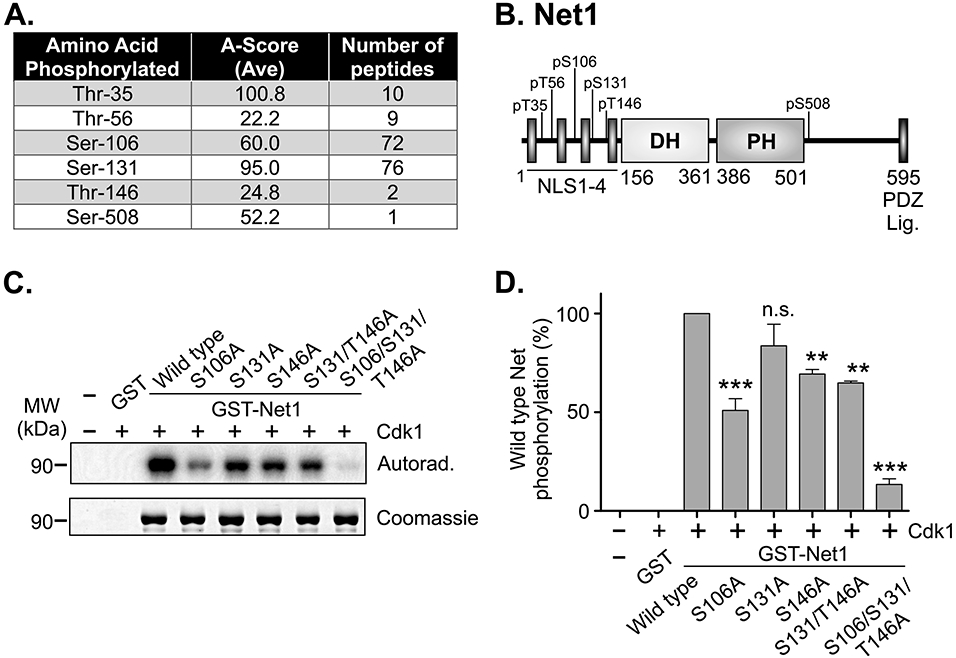
(A) Table of phosphopeptides from LC/MS/MS analysis of recombinant mouse GST-Net1 phosphorylated by purified Cdk1-Cyclin B in vitro. Note that T35 is not conserved in human Net1. (B) Schematic of the domain structure of Net1, and Cdk1 phosphorylation sites. (C) In vitro Cdk1-dependent phosphorylation of recombinant GST-Net1 proteins with the mutations shown. Phosphorylation was detected by incorporation of 32P-labeled phosphate and autoradiography. Equal loading of proteins was monitored by Coomassie staining. Shown is a representative experiment. (D) Quantification of phosphorylation of GST-Net1 proteins by purified Cdk1 in vitro. Shown is the average of 3 independent experiments. Errors are standard error of the mean (SEM). ** = p<0.01; *** = p<0.001; n.s. = not significant.
