Figure 3. Substitution of the Cdk1 phosphorylation sites does not affect Net1 stability.
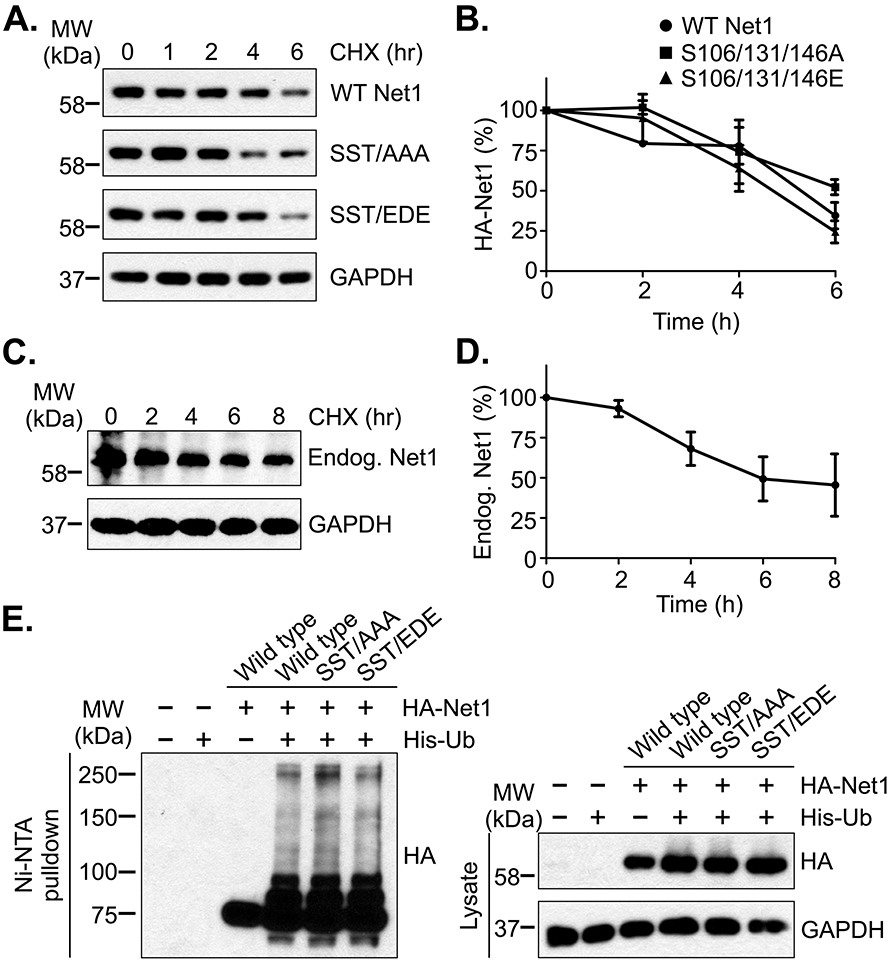
(A) Stability of wild type and mutant HA-Net1 proteins in prometaphase. HeLa cells were transfected with the Net1 expression vectors shown, synchronized in prometaphase, and protein synthesis was halted by addition of cycloheximide (CHX) for the times shown. Cells were lysed in SDS-containing buffer and HA-Net1 expression was examined by western blotting. Shown is a representative experiment from 3 independent experiments. (B) Quantification of transfected Net1 stability in pro-metaphase. Errors are SEM. (C) Stability of endogenous Net1 in prometaphase. HeLa cells were synchronized in prometaphase and cycloheximide was added for the times shown. Endogenous Net1 expression was detected by western blotting. Shown is a representative experiment from 4 independent experiments. (D) Quantification of endogenous Net1 stability in pro-metaphase. Errors are SEM. (E) Substitution of the Cdk1 phosphorylation sites in Net1 does not affect its ubiquitylation. HeLa cells were transfected with the HA-tagged Net1 expression plasmids shown, plus a vector encoding 6xHis-Ubiquitin, synchronized in prometaphase, and treated with MG132 for 1 hour. The cells were then lysed in a urea-containing buffer, ubiquitylated proteins were isolated using Ni-NTA-agarose, and ubiquitylation was assessed by western blotting for the HA-epitope. Shown is a representative experiment from 3 independent experiments.
