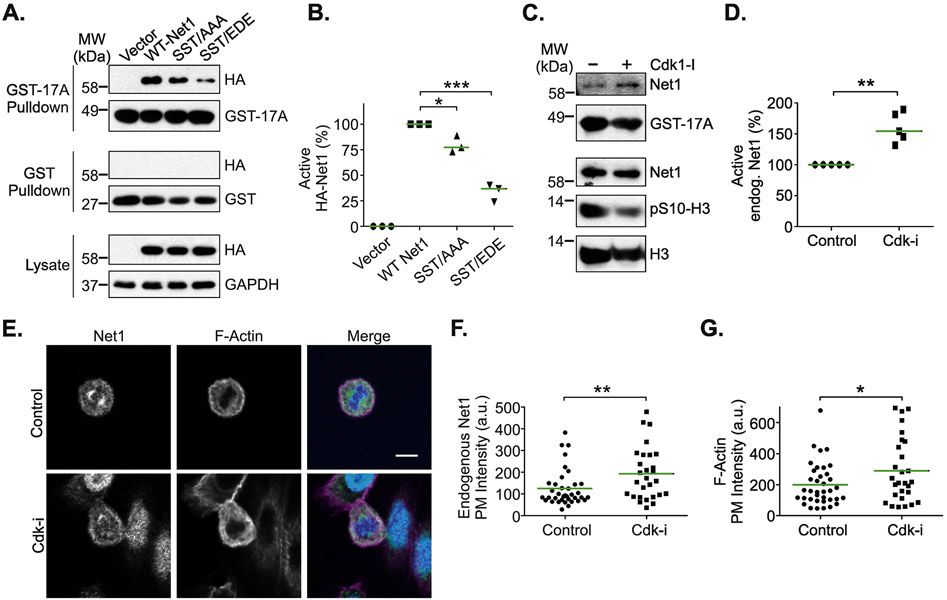
Figure 4. Effects of Cdk1 on Net1 activity and localization.
(A) Substitution of the Cdk1 phosphorylation sites downregulates Net1 activity. HeLa cells were transfected with the HA-Net1 expression plasmids shown, synchronized in prometaphase, and active Net1 proteins were isolated by GST-A17RhoA pulldown (top blots). Lysates were also probed with GST alone as a control (middle blots). Proteins present in the lysates are shown in the bottom blots. Shown is a representative experiment. (B) Quantification of the activation state of Net1 proteins in prometaphase cells. Bars are median values. * = p<0.05; *** = p<0.001. (C) Treatment of prometaphase cells with rosocovitin (10 μM, 2 hrs) increases endogenous Net1 activation, as measured in GST-A17RhoA pulldowns. Shown is a representative experiment from 5 independent experiments. (D) Quantification of endogenous Net1 activation state in prometaphase cells treated with roscovitin. Bars are median values. ** = p<0.01. (E) Treatment of cells with the Cdk inhibitor roscovitine (10 μM, 2 hrs) causes increased plasma membrane localization of endogenous Net1 and increased cortical F-actin formation. Cells were fixed and stained for endogenous Net1 (green), DNA (blue), and F-actin (magenta), and imaged using confocal microscopy. Shown are representative z-plane images. Scale bar = 10 μm. (D) Quantification of endogenous Net1 intensity at the plasma membrane in vehicle and roscovitin (Cdk-i) treated, prometaphase cells. Each point represents one cell. Data are aggregated from 3 independent experiments. Bars are median values. ** = p<0.01. (E) Quantification of plasma membrane F-actin staining in vehicle and roscovitin treated, prometaphase cells. Each point represents one cell. Data are aggregated from 3 independent experiments. Bars are median values. * = p<0.05.