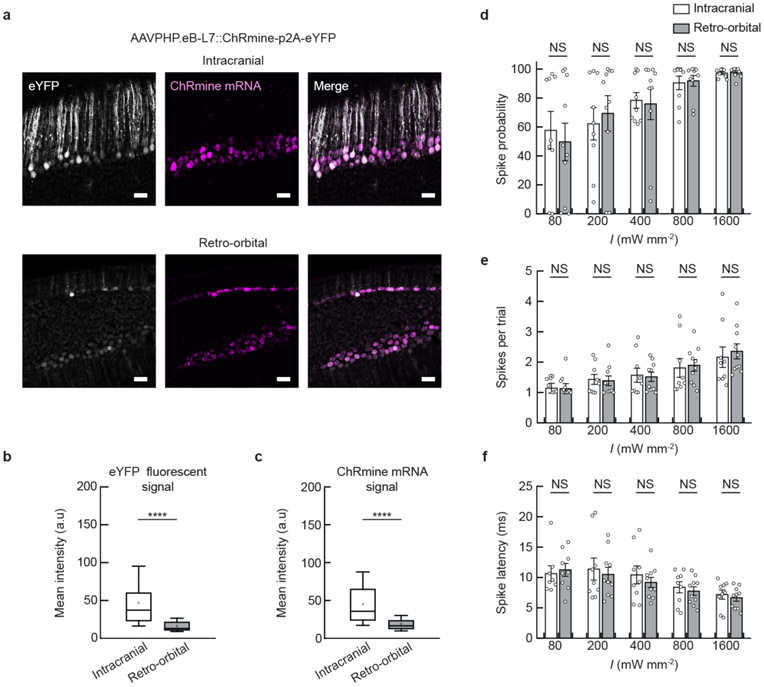
Extended Data Fig. 8 ∣. Comparison of expression level and photoactivity of ChRmine-expressing neurons targeted by intracranial or retro-orbital delivery.
a, Confocal images of Purkinje neurons expressing eYFP (white) co-labeled with in situ hybridization for ChRmine mRNA (magenta). Neurons were targeted by AAVPHP.eB-L7::ChRmine-p2a-eYFP by direct or retro-orbital injection. Scale bar: 100 μm. Box plot of mean intensity signal from individual neurons for (b) eYFP and (c) ChRmine mRNA (n = 2 mice, 60 neurons per group; two-sided unpaired t-test, P = 6.7e-10 (eYFP), 5.2e-10 (ChRmine mRNA)). The mean signal of neurons targeted by retro-orbital injection was 42% (eYFP) and 34% (ChRmine mRNA) relative to the signal from direct injection, indicative of the lower multiplicity of infection. Each box denotes lower (25%) and upper (75%) quartile with median (line) and mean (square) and whiskers depict the 10-90% range for neurons quantified. In vivo extracellular recordings at 3 mm from skull following transcranial stimulation from neurons expressing ChRmine targeted by intracranial (not filled) or retro-orbital (gray) injections plotted as: probability of one or more evoked spikes (d); number of spikes per pulse (e); and latency to the first spike (f) across the population of neurons as a function of irradiance (I) delivered at 10 Hz with 5-ms pulse width. No statistically significant difference was observed in single-unit response between the viral delivery methods (n = 9 units from 2 mice (intracranial) and n = 10 units from 2 mice (retro-orbital); two-way ANOVA with Bonferroni post hoc tests: intracranial vs. retro-orbital F(1,85) = 0.002, P = 0.96 (d); F(1,83) = 0.05, P = 0.82 (e) and F(1,83) = 0.65, P = 0.42 (f). Note one neuron in each group exhibited no spike response at 80 mW mm−2 and as such has no corresponding spike count or latency at this irradiance). ****P < 0.0001; NS, not significant. Data are mean ± sem.