Figure 6. SKIP is Negatively Regulated by its C-Terminal Domain.
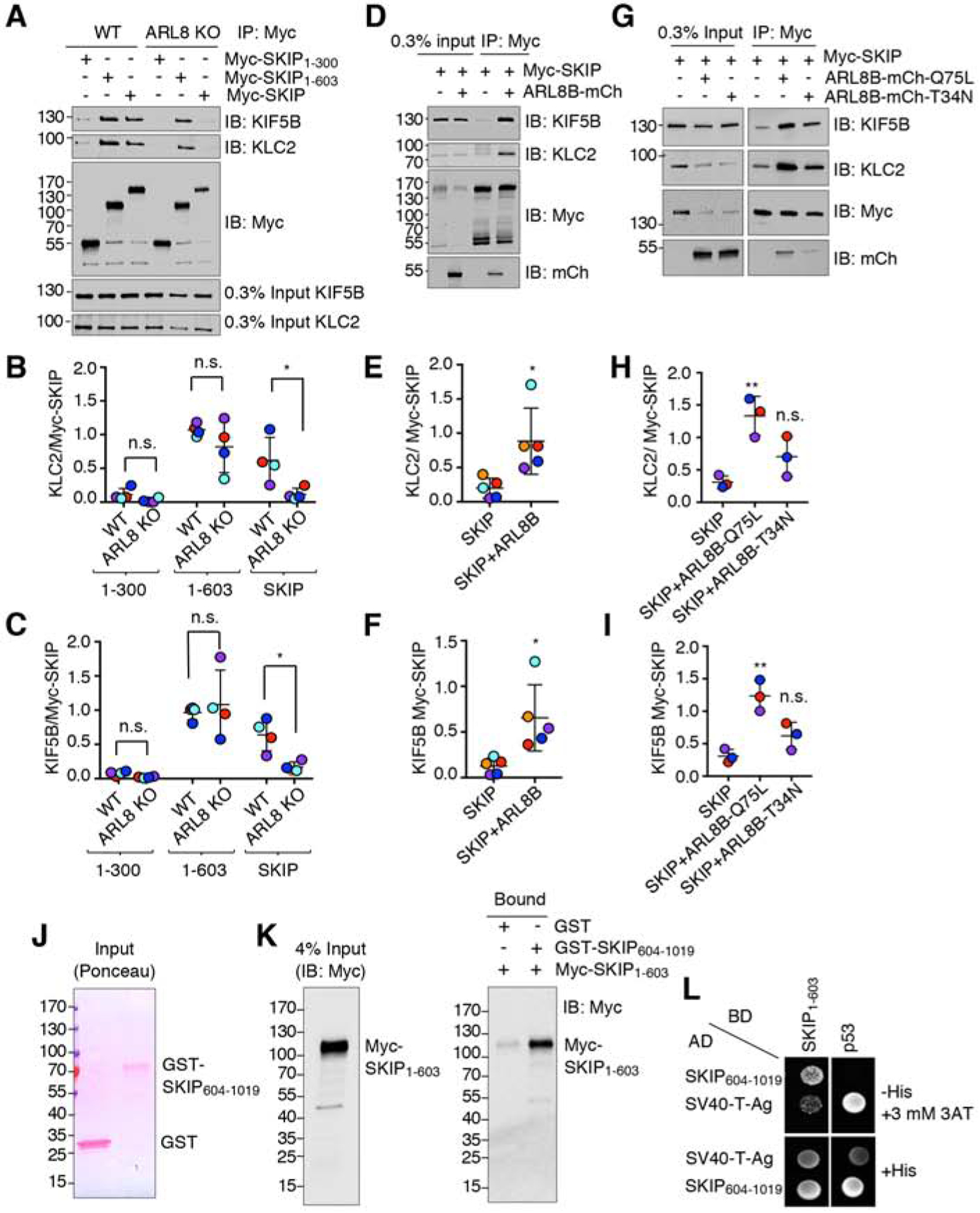
(A) Co-immunoprecipitation of full-length and truncated Myc-SKIP constructs with kinesin-1 in WT and ARL8-KO HeLa cells. Cells were transfected with plasmids encoding the indicated Myc-SKIP constructs, subjected to immunoprecipitation using an antibody to the Myc tag, followed by SDS-PAGE and immunoblotting with antibodies to the endogenous KIF5B and KLC2 subunits of kinesin-1 and the Myc tag.
(B,C) Quantification of experiments such as that shown in panel A. Signal intensity was quantified by Image J. The ratio of KLC2 (B) or KIF5B (C) to Myc-SKIP in the immunoprecipitates was calculated, and relative Myc-SKIP binding in WT HeLa was normalized to 1. Horizontal lines represent the mean ± SD from 4 independent experiments. Independent experiments are color-coded. Statistical significance was calculated using two-way ANOVA followed by multiple comparisons using Sidak’s test. Not significant (n.s.) P>0.05, *P<0.05.
(D) Co-immunoprecipitation of endogenous KIF5B and KLC2 with Myc-SKIP in the absence or presence of ARL8B-mCherry overexpressed by transfection in HEK293T cells. Cell extracts were subjected to immunoprecipitation using an antibody to the Myc tag followed by SDS-PAGE and immunoblotting with antibodies to the endogenous KIF5B and KLC2 subunits of kinesin-1 and the Myc tag.
(E,F) Quantification of experiments such as that shown in panel D. Signal intensity was quantified by Image J. The ratio of bound KLC2 (E) or KIF5B (F) to bound Myc-SKIP signals was calculated. Horizontal lines represent the mean ± SD of 5 independent experiments consisting of 6 and 9 replicates per sample for SKIP and SKIP+ARL8, respectively. Independent experiments are color-coded. Statistical significance was calculated using Student’s t-test. Not significant (n.s.) P>0.05, *P<0.05.
(G) Co-immunoprecipitation of endogenous KIF5B and KLC2 with Myc-SKIP in the absence or presence of ARL8B-mCherry T34N (GDP-bound) and Q75L (GTP-bound) overexpressed by transfection in HEK293T cells. Cell extracts were subjected to immunoprecipitation using an antibody to the Myc tag followed by SDS-PAGE and immunoblotting with antibodies to the endogenous KIF5B and KLC2 subunits of kinesin-1 and the Myc tag.
(H,I) Quantification of experiments such as that shown in panel G. Signal intensity was quantified by Image J. The ratio of bound KLC2 (H) or KIF5B (I) to bound Myc-SKIP signals was calculated. Horizontal lines represent the mean ± SD from 3 independent experiments consisting of 7 replicates per sample. Independent experiments are color-coded. Statistical significance was calculated by one-way ANOVA followed by multiple comparisons using Tukey’s test. Not significant (n.s.) P>0.05, *P<0.05, **P<0.01.
(J,K) Pulldown by purified, recombinant GST and GST-SKIP604–1019 of Myc-SKIP1–603 expressed by transfection in HEK293T cells. GST and GST-SKIP604–1019 were immobilized on glutathione-Sepharose and incubated with lysates of Myc-SKIP1–603 -expressing HEK293T cells. Samples were analyzed by SDS-PAGE; GST proteins were visualized by Ponceau staining (J) and Myc-SKIP1–603 by immunoblotting with antibody to the Myc epitope (K). In A, D, G, J and K, numbers on the left indicate the positions of molecular mass markers (in kDa).
(L) Yeast two-hybrid analysis of the interaction of SKIP604–1019 fused to the β-Gal activation domain (AD) with SKIP1–603 fused to the β-Gal DNA-binding domain (BD). Growth in the absence of histidine (−His) indicates binding between the proteins. Three mM 3AT was added to reduce non-specific growth.
