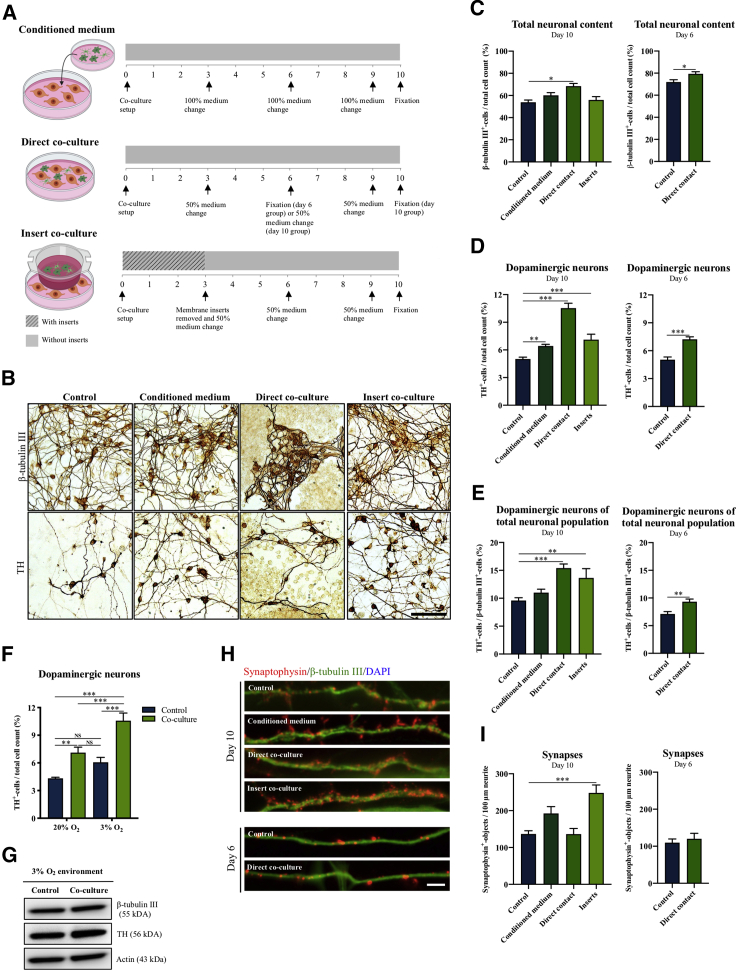
Figure 1.
Increased Dopaminergic Differentiation of NSCs Using Different Microglia Co-culture Setups
(A) hVM1-Bcl-XL NSCs were either exposed to BV2 microglia-conditioned medium, directly co-cultured with BV2 microglia (physical contact), or indirectly co-cultured (separated by semi-porous membrane inserts).
(B–E) Immunocytochemical staining and quantification of differentiated neurons for (C) β-tubulin III+ neurons/total cell count, (D) TH+ neurons/total cell count, and (E) the number of TH+ neurons/β-tubulin III+ neurons. Scale bar: 100 μm. One-way ANOVA, Dunnett's multiple comparison test with reference to control. Day 10: control, n = 23, N = 6; conditioned medium, n = 16, N = 4; direct contact, n = 6, N = 2; inserts, n = 13, N = 4. Day 6: control, n = 14, N = 4; direct contact, n = 14, N = 4.
(F and G) TH+ neurons/total cell count and Western blotting for β-tubulin III and TH in differentiated hVM1-Bcl-XL NSCs cultures after combining the indirect co-culture setup with physiological O2tension (3% O2). Two-way ANOVA, Tukey's multiple comparison test. Control, n = 14, N = 4; co-culture, n = 13, N = 4.
(H and I) Synaptophysin+ objects/100 μm neurite. Scalebar: 5 μm. One-way ANOVA, Dunnett's multiple comparison test with reference to control. Day 10: control, n = 6, N = 2; conditioned medium, n = 6, N = 2; direct contact, n = 4, N = 2, inserts, n = 6, N = 2. Day 6: control, n = 4, N = 2; direct contact, n = 4, N = 2. Mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, NS = not significant.
See Figures S1 for additional data.