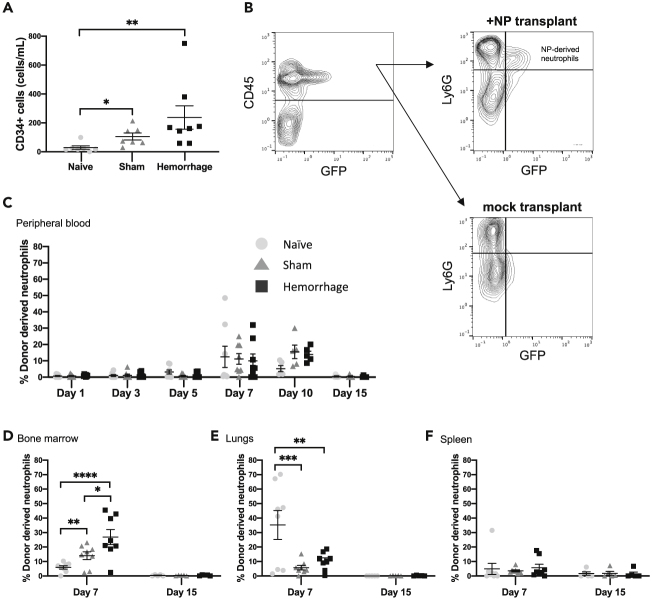
Figure 5.
Hemorrhage Enhances Transient Bone Marrow Engraftment of NPs
Mice were subjected to either hemorrhagic shock, sham hemorrhage, or no procedure (naive). In (B)–(F), mice then received transplant with or without NPs.
(A) Twenty-four hours post hemorrhage, the degree of CD34+ cells mobilized into the circulation was analyzed by flow cytometry. Data are expressed as the mean number of CD34+ cells per milliliter ± SEM. Data were fit to a lognormal distribution, analyzed by GLMs, and the Holms test was used to adjust for multiple comparisons.
(B and C) (B) Representative gating strategy for determining the degree of donor-derived neutrophil chimerism. Leukocytes were identified by CD45 expression, then neutrophils were identified by Ly6G expression. Donor-derived neutrophils were then distinguished from host by GFP expression, with gating performed according to mice receiving mock transplant. The degree of neutrophil chimerism in the (C) peripheral blood (n = 6 for hemorrhage day 5, n = 7 for sham day 5, n = 5 mice per condition for days 10 and 15, n = 8 for the remaining conditions). All flow cytometry data taken from blood represent a repeated measure of the same mice over time from day 1–7 and then a second cohort of mice from day 10–15.
(D–F) (D) Bone marrow (n = 8 mice per condition), (E) lungs (n = 8 mice per condition), and (F) spleen (n = 8 mice per condition) was determined over the course of 15 days. Each replicate of bone marrow, lungs, and spleen flow cytometry data represents repeated measures of the same mouse in different tissues. Distinct mice were used for the 7- and 15-day timepoints. Data are expressed as mean percentage of donor-derived neutrophils ±SEM. Data were fit to a binomial distribution, analyzed by GLMs, and the Holms test was used to adjust for multiple comparisons. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.