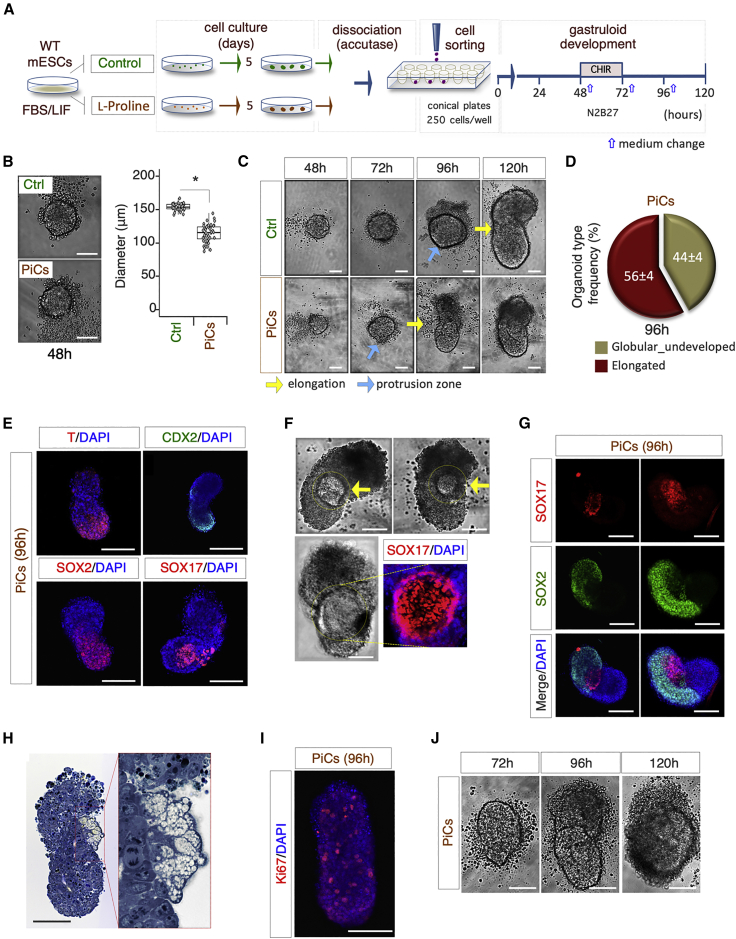
Figure 5.
Proline-treated ESCs Are Competent for Gastruloid Formation
(A) Schematic representation of experimental design.
(B) Representative bright-field pictures (left) of control (Ctrl) and PiC-derived aggregates (48 h) and boxplot diagram (right) of diameter distribution (n = 3; 45 gastruloids/condition; ∗p < 0.01; bar, 100 μm).
(C) Representative bright-field images of globular aggregate-to-elongated gastruloid transition of PiCs and Ctrl cells (bar, 100 μm). Light blue and yellow arrows indicate the protrusion zone and the ovoidal-to-elongated shape transition, respectively.
(D) Pie chart quantification of PiC-derived organoid type frequency at 96 h. Data are expressed as mean ± SD (n = 3; 60 gastruloids analyzed).
(E) Representative confocal images of PiC-derived gastruloids stained with T/BRA, SOX2, SOX17 (red), and CDX2 (green). Nuclei were counterstained with DAPI (bar, 200 μm).
(F) Representative bright-field and confocal images of PiC-derived gastruloids (96 h) showing the SOX17-positive area (yellow arrows) (bar, 100 μm).
(G) Representative confocal images of PiC-derived gastruloids stained with SOX17 (red) and SOX2 (green). Nuclei were counterstained with DAPI (bar, 100 μm).
(H) Representative pictures of toluidine blue-stained sections of PiC-derived gastruloids (96 h). Picture enlargement shows a differentiated area (bar, 100 μm).
(I) Representative confocal image of PiC-derived gastruloid stained with Ki67. Nuclei were counterstained with DAPI (bar, 100 μm).
(J) Representative bright-field images of PiC-derived organoids at the indicated time points (bar, 100 μm).
See also Figure S4.