Abstract
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infections in children worldwide, with the most severe disease occurring in very young infants. Despite half a century of research there still are no licensed RSV vaccines. Difficulties in RSV vaccine development stem from a number of factors, including: (a) a very short time frame between birth and first RSV exposure; (b) interfering effects of maternal antibodies; and (c) differentially regulated immune responses in infants causing a marked T helper 2 (Th2) immune bias. This review seeks to provide an age-specific understanding of RSV immunity critical to the development of a successful pediatric RSV vaccine. Historical and future approaches to the prevention of infant RSV are reviewed, including passive protection using monoclonal antibodies or maternal immunization strategies versus active infant immunization using pre-fusion forms of RSV F protein antigens formulated with novel adjuvants such as Advax that avoid excess Th2 immune polarization.
Keywords: Respiratory Syncytial Virus, RSV, vaccine, adjuvant, infant, neonate
Introduction
Respiratory syncytial virus (RSV) (see Figure 1)1 is the leading cause of pediatric acute lower respiratory tract infections (ALRI), with the incidence peaking at 2–4 months of age2 and most cases of severe RSV occurring in infants <6 months of age.3 Using a risk factor-based model and 2015 population estimates in 132 developing countries, the RSV Global Epidemiology Network estimated ~ 33.1 million annual episodes of RSV-ALRI, 3.2 million hospital admissions, and 59,600 in-hospital deaths among children under the age of 5 years.4 In infants younger than 6 months of age, RSV-ALRI was estimated to be responsible for 1.4 million hospital admissions and 27,300 in-hospital deaths.4 In a retrospective case series, the median age for global RSV-related deaths was between 5 and 7 months of age.5 Overall, about 45% of hospital admissions and in-hospital deaths due to RSV-ALRI occur in children younger than 6 months. Accompanying the heavy burden of RSV clinical disease is the large financial burden, with projected annual costs attributable to RSV infection of $342 million in the United States alone.6 In the absence of an effective treatment option for RSV disease7 there is an urgent need for early-life RSV prevention strategies to help reduce RSV-associated morbidity and mortality.
Figure 1.
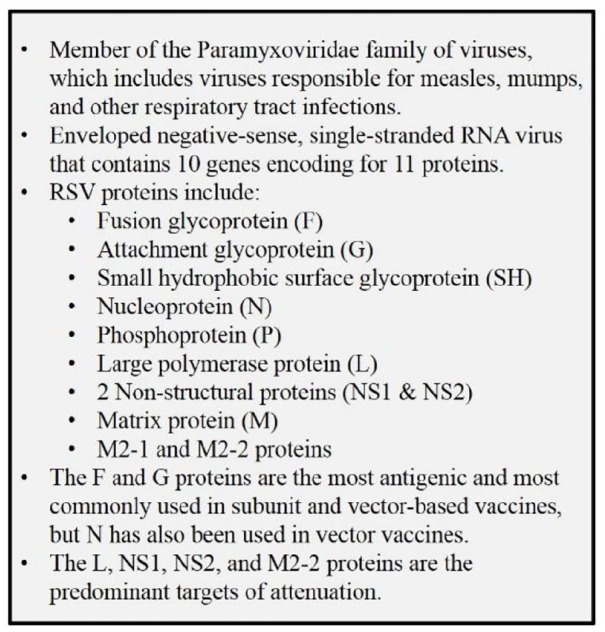
Key facts on respiratory syncytial virus (RSV).1
Despite the clear need, the development of a safe and effective RSV vaccine for young children has met with significant barriers. These include difficulty in the ability to generate protective long-term immune memory in young children and concerns regarding the potential for vaccine enhanced respiratory disease (ERD) when vaccinated children contract RSV infection. The lack of long-term immunity is a particular problem for RSV and individuals can be infected repeatedly throughout their lives. RSV infection does not induce long-term immunity even in adults. The problem of ERD was first recognized during clinical trials that took place in the 1960s to test the efficacy of a formalin-inactivated RSV vaccine (FI-RSV) formulated with alum adjuvant.8–11 In a study of 2–7-month-old children, the FI-RSV vaccinated children developed an enhanced form of RSV disease, now referred to as ERD, following natural RSV infection. This resulted in increased rates of hospitalization of 80% of the vaccinees, with two deaths.11 All vaccinated infants had measurable RSV antibody after immunization but the FI-RSV vaccine failed to provide any protection and instead caused ERD. This clinical trial failure impeded subsequent RSV vaccine development for many years; only recently have strategies been advanced to address these safety concerns, as will be discussed later in this review.
In addition to the issue of ERD, viable pediatric RSV immunization strategies must also overcome reduced infant immune responsiveness to vaccination, including the potential inhibitory effects of maternally derived RSV antibodies. Infants, particularly those that are born prematurely, possess an immature innate and adaptive immune system that leaves them more vulnerable to pulmonary infections (see Figure 2). Neonates have a reduced ability to generate effective, long-lived adaptive memory responses following immunization, attributed to a predisposition towards tolerogenic immune responses. Newborn cord-blood dendritic cells (DCs) and monocytes stimulated with Toll-like receptor (TLR) agonists demonstrate a preference to T helper (Th)-2 cytokine responses with reduced production of Th-1 cytokines.12 Moreover, neonatal lymphocytes exhibit a high proportion of recent thymic emigrants,13 which are biased towards the Th2 effector lineage.14 Humoral immunity of the infant is disadvantaged as there is a low frequency of somatic mutations, thereby leading to lower avidity and less antibody diversity.15 In the United States hepatitis B vaccine is the only vaccine recommended for administration at birth and still requires subsequent booster doses.16,17 Outside the US, Bacillus Calmette–Guerin (BCG) and oral polio vaccine (OPV) are live vaccines given at birth. However, OPV still requires three additional childhood boosters. While BCG protects infants from serious complications of meningitis and disseminated tuberculosis, it does not protect from primary tuberculosis. Collectively, these examples highlight the difficulties of developing vaccines to protect very young infants from infectious diseases.
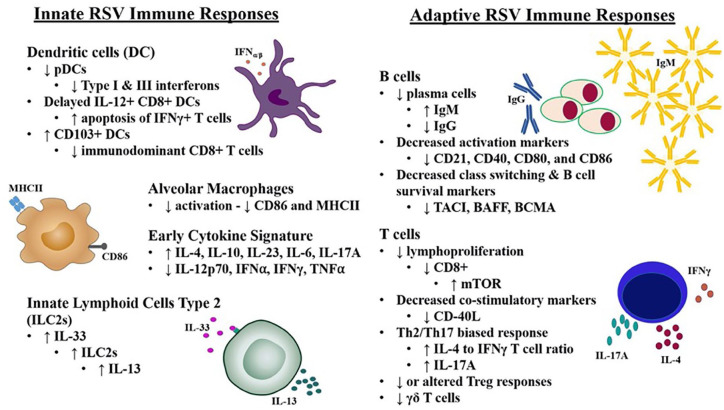
Figure 2.
Differences in immune responses to RSV between infants and adults. Data from RSV-infected infants and animal models demonstrate infant immune responses are manifested by reduced anti-viral type I/II interferon (IFN) production and increased T helper (Th)-2 and anti-inflammatory cytokine production when compared to adults. The adaptive immune response of infants is characterized by reduced activation and IgG class switching of B cells with higher production of IgM compared to IgG from a reduced plasma cell population. Neonatal T-cell populations are also altered and demonstrate reduced proliferation, decreased expression of co-stimulatory markers, and a higherinterleukin (IL)-4/IL-17A to IFNγ ratio
BAFF, B-cell activating factor of the tumor necrosis factor family; BCMA, B-cell maturation antigen; mTOR, mammalian target of rapamycin; pDC, plasmacytoid dendritic cells; RSV, respiratory syncytial virus; TACI, transmembrane activator and calcium-modulating cyclophilin ligand interactor.
Our understanding of RSV immunity comes from more than 50 years of clinical research and use of animal models. Neutralizing antibody has been shown to be an important correlate of protection.18–21 More recently, the superiority of neutralizing antibodies targeting RSV pre-fusion protein has been demonstrated.22–24 Studies describing mucosally-associated immunity, including RSV-specific memory B cells,24 breast milk IgG,23 and nasal IgA,25 as well as interferon (IFN)-γ-producing RSV-specific CD8+ T cells have broadened our understanding of protective immunity.26–31 These findings highlight the importance of considering correlates of protection beyond serum neutralizing antibodies in RSV vaccine research. Although the role of eosinophils in causing immunopathology is controversial, eosinophilia is an indication of overexuberant Th2 immunity, which has been associated with RSV immunopathology.32,33 The recent characterization of the pre-fusion conformations of the RSV F protein has helped advance immunization. This review will discuss the latest approaches to the development of infant RSV vaccines, including the use of novel adjuvants to overcome neonatal immune hypo-responsiveness.
Infant RSV immune responses
Innate responses
The infant immune response to RSV is characterized by suboptimal type I and II interferon responses.34–36 In murine models of RSV infection, infants produced significantly less IFN-α, β and γ than similarly infected adult mice.34,37,38 This blunted type I interferon response mirrored the decreased plasmacytoid dendritic cell (pDC) frequency in RSV-infected infant murine lungs.34 The absence of these crucial anti-viral cytokines during infant RSV exposure severely impacts the ability of infants versus adults to mount effective anti-RSV responses.34,35,39 Furthermore, supplementing these type I/II interferons during early RSV infection in an infant murine model of RSV improves the immune response on re-infection, resulting in reduced airway hyperresponsiveness and lung pathology.34,35 These studies highlight the importance of type I/II interferons in early RSV protection, and successful pediatric vaccine strategies should ideally overcome this deficit.
In addition to the blunted type I/II interferon responses, infants also display elevated production of anti-inflammatory cytokines following exposure to RSV, which are typically associated with greater disease severity.38,40 In murine models of RSV infection, infants expressed more interleukin (IL)-4 and IL-10 shortly after RSV exposure.38 This suggests that the early production of these cytokines skews the infant lung towards a Th2 environment, which by suppressing Th1 anti-viral T-cell responses, may lead to less effective viral control and clearance. Similarly, studies conducted in human neonatal blood show robust production of Th2 cytokines and reduced production of Th1 cytokines following TLR stimulation. Neonatal whole blood immune cells, when compared to similarly stimulated adult blood immune cells, produced fewer Th1-promoting cytokines (ex. IL-12p70, IFNα, IFNγ, and tumour necrosis factor (TNF)-α) while expressing more IL-23, IL-6, IL-1b, and IL-10, well-known promoters of Th17 and anti-inflammatory responses.11 Even in low-resource settings known to have greater infectious pressure, most TLR agonists (TLRs 1–9) induced production of TNFα, IL-1β, IL-6, and IL-10 in cord blood.41 The greatest TNFα and IFNγ responses were produced from cord blood samples following stimulation with a TLR7/8 agonist. However, one striking finding from TLR stimulation studies done in blood from African41 and Papua New Guinean42 infants was the robust and persistent IL-10 response. RSV activates several Toll-like receptors (TLRs),43–45 and single nucleotide polymorphisms (SNPs) in multiple TLRs have been associated with RSV disease severity.46,47 The differential response to TLR stimulation in infants should be taken into consideration when designing pediatric RSV vaccines. Furthermore, overcoming anti-inflammatory bias may be especially important in areas with high rates of infections, e.g. parasites and helminths, that induce Th2-dominant immune responses.
Differential cytokine production in infants parallels many other differences in cell populations and innate immune activation pathways in infants. As the predominant immune cell present in the naïve airspace, alveolar macrophages (AMs) play an important role in the early immune response to RSV, characterized by rapid AM activation and phagocytosis of the virus. However, infant mice mount an immature AM response, with delayed upregulation of major histocompatability complex (MHC)-II and CD86.38 Exogenous intranasal delivery of IFNγ overcame infant murine AM immaturity, expediting activation and viral clearance.48 Notably, depletion of infant murine AMs resulted in higher viral titers indicating a role for AMs in RSV control.48
Neutrophils are an innate immune cell commonly identified in clinical bronchoalveolar lavage (BAL) samples obtained from pediatric patients infected with RSV.49–51 They have been reported to represent approximately 70–85% of cells recovered from clinical samples obtained from infants and children with RSV bronchiolitis. Interestingly, a correlation between IL-8, a chemokine integral in the recruitment of neutrophils, and RSV clinical severity has been established in pediatric patients.51 However, cell counts from bronchial secretions do not correlate with RSV clinical severity51 despite increased airway neutrophil frequency following RSV infection.52 Therefore, although neutrophils are commonly found in infants and children with severe RSV, their precise role in the pathological response is unclear.
With the well-characterized Th2 cytokine environment of the infant airspace, it is not surprising that type 2 innate lymphoid cells (ILC2s) play an important early role in shaping pediatric responses to RSV.53,54 At baseline, infant mice have significantly more ILC2s in their lungs than adults, and on RSV challenge, these cells quickly expand and produce IL-13. This quick ILC2 response is mediated by epithelial cell release of IL-33, which is significantly higher in the lungs of infant compared to adult mice.53 Importantly, elevated populations of ILC2s and concentrations of IL-33 and IL-13 were identified in the nasal aspirates of infants hospitalized with severe RSV infection.53,54
In addition to aberrations in AM function and enhanced ILC2 responses, neonates also display altered DC behaviour.55–57 In early life, infant mice show delayed development of IL-12-producing CD8+ DCs, leading to the apoptosis of IFNγ-producing Th1 cells.55 This reduction in Th1 immunity compromises anti-viral immune responses. Furthermore, infant mice show a predominant CD103+ DC phenotype in draining lymph nodes following RSV infection, with reduced immunodominant Kd M282–90-specific CD8+ T cells compared to adult mice.57 As DCs play a crucial role in bridging the innate and adaptive responses, knowledge of how neonatal DCs differ from adult DCs needs to be factored into the design of RSV vaccines aimed at protecting infants. Hence, many aspects of the innate immune system display age-dependent differences that affect infant immunity and predispose infants to more severe RSV infection, and less efficient vaccine responses. Understanding these differences will be critical to the development of effective pediatric RSV vaccines.
Infant B-cell responses to RSV
The infant B-cell response to purified antigens is generally weak compared to adults. Nonetheless, in vivo data suggest that B cells are extensively recruited and activated in response to RSV infection, even in infants. Quantitatively, B cells are increased in the peripheral blood of RSV-infected infants aged 1–12 months compared to healthy controls,58 yet the functional capacity of these B cells has not been extensively investigated. Reed et al. analyzed post-mortem lung samples from fatal infant RSV cases and found that B cells were present in the lower respiratory tract, while CD4+ and CD8+ T cells were essentially absent.59 As RSV-specific antibodies were detected in nasopharyngeal secretions, a T-cell independent antibody production process dependent on local innate immune factors may be a potential explanation for this observation. In both human and murine models of RSV, low levels of antibodies are produced following antigen exposure in infants, with less efficient affinity maturation.60–62 Following RSV exposure in human infants, plentiful IgM is produced by B cells whereas IgG is produced in relatively low quantities compared to adults, despite similar levels of the B-cell activation markers, CD69 and CD86.63 The relatively low antibody response to infection suggests infants may elicit predominantly short-lived effector B-cell responses rather than production of long-lived plasma cells. Indeed, this was observed in a neonatal mouse model of tetanus toxoid vaccination, in which early life immunization led to the development of significantly fewer bone marrow plasma cells compared to immunized adult mice.64 However, the ability to mount a humoral response to antigen progresses rapidly within the first 3 months of life.65 By 6 months of age, significant increases in the quantity of antibody production is notable in humans following RSV exposure.66 Younger age is also associated with differences in RSV epitope recognition by antibodies, with responses in children older than 6 months starting to resemble adult-like responses.67 Many of these maturational changes in B-cell development coincide with the natural decay of maternal antibodies, which may also influence RSV-specific B-cell responses in infants.
Several differences in infant immunology offer potential explanations for the decreased overall B-cell response to viral antigen. Decreased strength of B-cell receptor signaling and decreased expression of B-cell receptors CD21, CD40, CD80, and CD86 may contribute to impaired production of IgG in humans.65,68 Neonatal B cells also show decreased expression of costimulatory receptors within the tumor necrosis factor receptor superfamily, which are relevant to B-cell survival and antibody class switching. Members of this family, including transmembrane activator and calcium-modulating cyclophilin ligand interactor (TACI), B-cell activating factor of the tumor necrosis factor family (BAFF), and B-cell maturation antigen (BCMA), were reduced in preterm neonatal cord blood compared to adults.69 Impaired T helper cell stimulation of antigen-specific B cells through CD40L–CD40 interactions may also contribute to this early-life B-cell phenotype, as decreased CD40L on T cells and CD40 on B cells have been observed in human infants compared to adults.68–70
Infant T-cell responses to RSV
The infant T-cell response to RSV in humans has been evaluated using peripheral blood samples from infants hospitalized with RSV-associated bronchiolitis. A more severe disease course was observed in infants with lower IFNγ and total T-cell counts at hospital admission compared to those with milder disease.71 T cells isolated from RSV-infected human infants showed significantly more production of IL-4 and less IFNγ than healthy controls, supporting the hypothesis that RSV elicits a Th2 skewed immune response.72 Furthermore, younger age at the time of infection has been associated with an allergy-associated T-cell response with increased proportions of Th2, Th9, Th17, and Th22 cells in infants and children.73 In one infant study that included nasal washes and lung tissue from post-mortem samples, nearly all cytokines measured, including IFNγ, IL-2, IL-4, and IL-17, were lower in infants infected with RSV than those infected with influenza,74 suggesting RSV may actively suppress T-cell immunity more than other respiratory tract infections. Notably, in fatal cases, CD8+ T cells were nearly absent in most lung samples.74 De Weerd et al. reported a non-significant trend to lower total CD8+ T-cell counts in RSV-infected human infants compared to controls.75 Other studies have, however, found evidence of a Th1 response accompanying the Th2 response to RSV infection in both humans and murine models.76–78 A limitation in the afore-mentioned studies is that most T-cell characterization has been performed with peripheral blood samples, which may not be representative of the T-cell response occurring in the RSV-infected airways. In addition, samples are generally restricted to a single time point during hospitalization (usually at admission) which precludes characterization of the kinetics of T-cell responses. Finally, most studies have included a range of ages, not restricting their assessment to young infants.
Animal models of RSV disease have allowed a more thorough characterization of T-cell responses to RSV. Infant models of RSV disease in mice reflect commonalities to in vivo human data, with a relatively reduced CD8+ T-cell response to RSV in infants compared to adults.79 Reduced CD8+ T-cell responses in infants may reflect the fact that RSV in human infants has been associated with increased mTOR gene expression with the use of an mTOR inhibitor leading to increased RSV-specific CD8+ T cells.80 Reduced lymphoproliferative responses to RSV in infants may also be related to deficiencies in IFNγ. In vitro and in vivo data in mice suggest that local delivery of IFNγ can increase the activation and proliferation of RSV-specific CD8+ T cells.79,81
CD4+ and CD8+ T-cell depletion studies in animal models have shed some light on their relative contributions to viral clearance and disease pathology, albeit with conflicting results. In a study by Graham et al., both CD4+ and CD8+ T cells appeared important to viral clearance in adult mice, with CD8+ T cells also suggested to play a role in lung immunopathology.27 CD8+ T-cell enhancement of lung pathology was also observed in another study following adoptive transfer of CD8+ T cells into RSV-infected adult mice, although no comparison was made to CD4+ T-cell transfer.26 T-cell adoptive transfer82,83 and T-cell depletion84 studies in mice suggested that CD4+ T cells also contribute to immunopathology. Depletion of CD8+ T cells in a mouse model of RSV infection was associated with increased eosinophilia suggesting CD8+ cells play a key role in preventing excess Th2 bias.28 In a study in adult mice in which anti-IL-4 was administered at the time of immunization with an FI-RSV vaccine adjuvanted with alum, the anti-IL-4 treated mice had significantly less weight loss and viral titers were reduced by five-fold, with RSV-specific cytotoxic CD8+ T-cell activity being augmented.85 This suggests IL4-producing CD4+ T cells play a key role in RSV pathogenesis with CD8+ T cells counterbalancing this. Age at first exposure to RSV also appears to play a critical role in pathophysiology of subsequent RSV infections. Mice infected with RSV at a younger age had increased total T-cell recruitment to the lung and greater severity of disease following re-challenge, with greater recruitment of Th2 cells and eosinophils.86 Hence, CD4+ T cells appear to make a greater contribution to RSV immunopathology than CD8+ T cells which if anything act to suppress excess Th2 responses. Facilitating optimal Th1 CD4+ and CD8+ T-cell responses to RSV through vaccination early in life may help to prevent subsequent immunopathology.
Other T-cell populations have been evaluated for their contribution to or amelioration of RSV immunopathology. T regulatory (Treg) cells are characterized by their anti-inflammatory activity, therefore the immunopathology observed in enhanced RSV disease may be due in part to excessive or impaired Treg responses. Adult mice depleted of Tregs prior to RSV exposure experienced increased severity of disease and a Th2 skewed response compared to control animals, demonstrating the importance of Tregs in controlling Th2-associated immunopathology.87 Infant mice infected with RSV developed a Treg population with a compromised suppressive function that instead produced Th2 cytokines.88 In agreement with pre-clinical data, a reduction in Tregs in the peripheral blood of infants hospitalized with RSV has been associated with RSV disease progression.89 The cytokine profile of the infants suggested a Th2 skewed response and a lack of IL-10 producing Tregs, resulting in an unbalanced immune response. Overall, both infant animal and human RSV data suggest that reduced IL-10 producing Tregs can result in an excess of Th2 cytokine producing CD4+ T cells. Moreover, FI-RSV vaccines formulated with the Th2-biasing alum adjuvant caused enhanced local recruitment of conventional CD4+ T cells accompanied by a profound loss of Tregs in adult mice.90 Adoptive transfer of CD4+ T cells harvested from FI-RSV immunized mice reduced Treg recruitment to the airway, resulting in increased immunopathology. Strategies to limit Th2 CD4+ polarization in response to immunization may ensure appropriate Treg activity, which could reduce the risk of immunopathology following RSV exposure.
Gamma-delta (γδ) T cells are yet another T-cell subset whose activity has been studied in relation to RSV immunopathology. In a study of hospitalized RSV-infected infants, a reduction in peripheral γδ T cells in the hospitalized infants with RSV was observed compared to controls, suggesting that γδ cells may play a role in RSV disease.74 Further investigation of γδ T cells in infant mice showed a higher total numbers of γδ T cells in the lung than adult mice early in RSV infection, but the γδ cells present had a less activated phenotype. The identified γδ T cells were largely responsible for the production of IL-17A.91 IL-17A concentrations in tracheal aspirates positively correlate with neutrophil recruitment to the airways of infant RSV patients.92 In the adult mouse model, CD4+ T cells were primarily responsible for IL-17A production.92
Passive immunization
Ineffective treatments (ex. ribavirin)93 combined with alterations in the neonatal immune response to RSV have prompted researchers to look for alternative means of protection. Passive immunization of vulnerable infants can provide immediate protection against RSV exposure and does not rely on vaccine immunogenicity in infants following direct immunization. This is an important consideration given the fact that infants were not able to mount an effective neutralizing antibody response before the age of 4 months following natural RSV infection.2 Passive immunity can be achieved through direct administration of prophylactic monoclonal anti-RSV antibodies or through maternal immunization.
Each approach is associated with its own benefits and drawbacks and each will be discussed in more detail below. Generally, immunoprophylaxis with anti-RSV monoclonal antibodies can be achieved with a known, effective dose. However, monoclonal antibodies require repeated administration to maintain protection due to the short antibody half-lives, proper timing of administration is required, recommendations for use are restricted to high-risk infants, therapy is costly, and there is a small risk of hypersensitivity reactions (less than one case per 100,000 patients).94 Passive protection through maternal immunization is beneficial because it has the potential for more complete coverage (i.e. development of IgA and multiple IgG subtypes, although IgG1 is the predominant subtype transferred placentally with IgA acquired through breastfeeding). Maternal immunization is also broadly applicable to every infant, provides protection at the time of birth, and there is no risk of hypersensitivity reactions. However, protection derived from maternal immunization is dependent on the immunogenicity of the vaccine in the mother, babies born prematurely may not be completely protected, and the timing of the maternal vaccination will affect the durability of the antibody levels in the infant.
Immuno-prophylaxis
Palivizumab, a humanized IgG1 monoclonal antibody produced by recombinant DNA technology, is a preventive treatment that targets a conserved epitope at the antigenic site II of the RSV fusion (F) protein resulting in inhibition of fusion activity and viral neutralization.95 It was licensed by the Food and Drug Administration (FDA) in 1998 (Figure 3) and is currently recommended for the prevention of RSV disease in high-risk infants, including those with congenital heart disease (CHD) and chronic lung disease by the American Academy of Pediatrics (AAP).96,97 Approval of palivizumab was based largely on the IMpact-RSV trial which enrolled 1501 children during the 1996–1997 RSV season and showed an absolute 5.8% reduction in RSV hospitalizations among high-risk infants that received palivizumab compared to the placebo group.98 A second randomized, double-blind, placebo controlled trial conducted from 1998 to 2002 also showed a reduction in RSV hospitalization rates from 9.7% to 5.3% among children with CHD.99 Despite its reported benefits of reducing the rate of RSV-associated hospitalizations, none of the five randomized, controlled trials demonstrated a significant decrease in RSV-attributable mortality. Moreover, economic analyses fail to demonstrate an overall savings in health care dollars in high-risk children owing, in part, to the difficulty in estimating cost per hospitalization among the highly varied rates of RSV hospitalizations within risk-stratified groups. Although the benefits of palivizumab have been shown for high-risk infants, significant costs estimated to be over $5500 for a 5-month course of therapy, have raised considerable debate regarding the overall value of palivizumab.5 Moreover, high cost, cold chain requirements, and the need for repeated injections remain significant barriers to delivering palivizumab to low-resource settings with high RSV morbidity and mortality.100,101
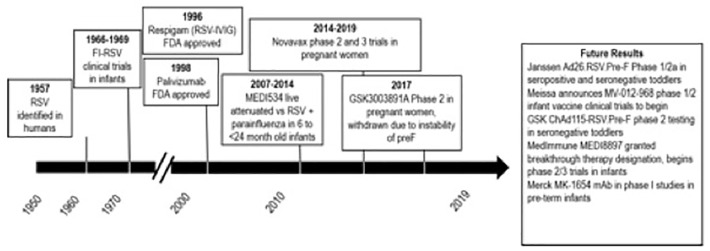
Figure 3.
Timeline of RSV - vaccine and monoclonal antibody development.
RSV, respiratory syncytial virus.
Recommendations from the AAP are continuing to evolve based on newer data and publications, including those that address palivizumab’s impact on the subsequent development of RSV-associated asthma and wheezing. Among these reports was a multicenter, double-blind, randomized, placebo controlled trial (MAKI) designed to assess the causal link between RSV infection and recurrent wheeze.102 Enrolled in this study were 429 otherwise healthy preterm infants, in which the total number of wheezing days was reduced by 61% in those children who received palivizumab versus placebo in the first year of life. In a single, assessor-blind, follow-up of the trial participants, children who had received palivizumab for RSV prevention as infants showed no change in the development of asthma compared to those who received placebo through 6 years of age.103 In a phase III, double-blind, placebo controlled, randomized trial in healthy term infants (⩽6 months of age), motavizumab (another anti-RSV monoclonal antibody) gave an 87% reduction in the proportion of infants admitted to the hospital with RSV compared to placebo.104 However, similar to the follow-up study of participants in the MAKI trial by Scheltema et al., there was no effect on rates of medically attended wheezing in children aged 1–3 years who received motavizumab compared to subjects who received placebo.
Newer preventive monoclonal antibodies attempt to address some of the barriers associated with palivizumab.105 MEDI8897 is an anti-RSV F monoclonal antibody in development that received a breakthrough therapy designation (BTD) from the FDA for the prevention of lower respiratory tract infection caused by RSV.106 The added benefit of MEDI8897 over palivizumab is its extended half-life, which is projected to protect at-risk children for a 5-month RSV season with a single 50 mg intramuscular dose as opposed to palivizumab monthly injections. Preterm infants and high-risk children with CLD and CHD are currently being recruited for a phase II/III study to evaluate the safety and tolerability of MEDI8897.107 Recent findings from a phase IIb study showed that MEDI8897 was safe compared to placebo with all-cause mortality and serious adverse event rates of 0.21% and 11.16%, respectively in MEDI8897-treated infants compared to 0.63% and 16.91% in the placebo group.108,109 In a similar endeavor to extend the half-life and reduce the number of injections required to protect infants during the RSV season, MK-1654, a broadly neutralizing human RSV antibody that targets the conserved site IV of the RSV F protein is being tested.110 A double-blind, randomized, placebo controlled study is currently recruiting healthy pre-term and full-term infants to evaluate the safety, tolerability, pharmacokinetics, and incidence of anti-drug antibodies following a single dose of MK-1654.111 Early studies show that the parent compound of MK-164, RB1 is approximately 50-fold more potent in vitro than palivizumab against both RSV A and B subtypes. MK-1654 is a modified version of RB1 whereby the Fc portion was altered to extend the half-life.110 These extended-life monoclonal antibodies may help overcome the need for repeated injections in at-risk children throughout the RSV season. With a reduction in the number of injections required, the deliverability of such a formulation to rural communities could be improved and the cost reduced.
Maternal immunization
The primary role of maternal antibodies is to attenuate infection severity and promote offspring survival.112 A number of clinical studies has shown that maternal neutralizing antibody levels correlate with protection from RSV disease.18,19,113–115 Clinically, maternal immunization has been embraced for protection from influenza, tetanus, diphtheria, and pertussis for both mothers and their offspring.116 Moreover, clinical data from full-term RSV-infected infants have suggested that higher maternal anti-RSV antibodies at the time of birth delay RSV infection.117 Pre-clinical studies have also demonstrated the protective potential of maternal RSV immunization. Intranasal immunization of female mice using a chimpanzee adenoviral vector expressing RSV F protein (AdC7-Fsyn) was protective for immunized mothers and their offspring.118 Offspring of AdCy7-Fsyn-immunized dams were protected from RSV challenge at 3 weeks of age through the transfer of RSV-specific IgG. Moreover, direct intranasal AdCy7-Fsyn immunization of offspring born to AdCy7-Fsyn-immunized mothers boosted their anti-RSV-specific IgG and was protective of RSV challenge. Importantly, this demonstrated that intranasal AdCy7-Fsyn immunization could be administered to mice in the presence of high maternal antibodies and still elicit boosted anti-RSV-specific IgG and protection from RSV challenge.
However, not all pre-clinical maternal immunization models demonstrate boosted anti-RSV IgG production when direct immunization occurs in the presence of maternal antibodies. In a maternal RSV immunization model using cotton rats, adult female rats were administered mono (palivizumab) or polyclonal (Respigam or GAMUNEX) anti-RSV antibody preparations 2 days prior to vaccination to simulate the presence of maternal antibodies. Dosing of mono and polyclonal antibody preparations was done to mimic average maternal anti-RSV antibodies found in the serum of human infants at 2 months of age. The animals were then immunized intranasally with SeVRSV, a live Sendai virus vaccine expressing the full-length RSV F gene.119 The animals were RSV challenged 3 months after immunization to allow the passively acquired antibodies time to wane and test the de-novo immunity elicited by SeVRSV vaccination. Despite high concentrations of passively acquired RSV neutralizing antibody, SeVRSV immunization conferred protection against RSV challenge. It is important to note, however, that (a) the safety of SeVRSV immunization was not described, (b) there was an indication that high levels of passively acquired RSV neutralizing antibodies reduced SeVRSV immunity following direct immunization and (c) the study was done to mimic a maternal immunization model but used adult animals, which may not accurately predict the immunogenicity of SeVRSV in infants.
The FI-RSV vaccine trials of the 1960s ended with disastrous results. Years of experimental evidence have demonstrated that ERD is largely mediated by Th2 cells.33,120 This was supported in a maternal model of FI-RSV vaccination, whereby FI-RSV immunized female mice developed severe Th2-associated immunopathology on RSV challenge, but their offspring were protected from RSV challenge by passively acquired maternal antibodies without evidence of increased immunopathology in the challenged infants.121 Despite formalin-inactivation of RSV altering RSV epitopes and resulting in poor antibody affinity maturation,122 offspring of FI-RSV immunized mothers were protected from RSV challenge as long as 4 weeks after weaning. Previous reports have suggested a role for pulmonary immune complex deposition and complement activation in ERD in FI-RSV vaccinated children.123 Significantly, RSV-challenged offspring of FI-RSV immunized female mice demonstrated no increases in peri-vascular or bronchial inflammation, mucus production, pulmonary eosinophil infiltration, or cytokine-secreting cell responses compared to primary RSV control animals.121 However, ERD was only evaluated in the offspring of FI-RSV immunized dams at 5 days post-RSV challenge. This time point may be too early to detect differences in T-cell responses elicited in the presence of maternal antibodies that may impact the development of ERD. Nevertheless, this study demonstrates that maternal immunization, even with a RSV vaccine with a poor safety record, can provide passive protection against RSV to their offspring without inducing early signals of immunopathology.
Clinical studies of maternal RSV vaccinations began in the early 2000s with the intramuscular (IM) administration of a RSV purified fusion protein 2 (PFP-2) subunit vaccine to women in their third trimester of pregnancy compared to placebo.124 The objectives of the study were to evaluate the safety and immunogenicity of PFP-2 in mothers and their offspring. Generally, PFP-2 immunization had a good safety profile in the mothers and was only associated with mild pain at the injection site. All infants were born healthy and there were no differences in peri or post-natal development between groups. Only 10% of vaccine recipients demonstrated four-fold increases in neutralization titers with increases generally seen in women with low pre-vaccination titers. Transplacental transfer of IgG was efficient (>100%) and anti-PFP-2 levels were four-fold higher in infants of vaccine recipients at birth, 2, and 6 months after delivery compared to placebo controls. Although the maternal PFP-2 vaccine lacked sufficient immunogenicity, it provided important proof of concept data demonstrating the efficiency of transplacental anti-RSV antibody transfer and a good safety profile.
Recently published data from phase II testing of the maternal RSV vaccine candidate, ResVax, [RSV fusion (F) protein nanoparticle adjuvanted with aluminum phosphate] in 50 healthy third-trimester pregnant women demonstrated good tolerability and no severe vaccine-related adverse events.125 Due to the small study group sample sizes and natural exposure to RSV, baseline levels of RSV antibodies were markedly different between sample groups. However, women who received RSV F vaccine generated antibody responses within 14 days of immunization and infants born to immunized mothers had higher levels of RSV-specific antibodies in their cord blood compared to placebo recipients. The efficiency of transplacental transfer was greater when the interval between immunization and delivery was ⩾30 days (110–120% of maternal serum) compared to infants born <30 days after maternal immunization (60–80% of maternal serum). The half-lives of RSV-specific maternal antibodies in infants were approximately 40 days. This study provided important safety data for a maternal RSV F vaccine candidate and demonstrated the importance of appropriate timing of maternal vaccination. By allowing an interval of ⩾30 days between immunization and birth, infants will be provided with the highest level of maternal antibody to protect them early in infancy when they are most vulnerable to severe RSV disease.
In July 2020, final results were released from the first ever phase III clinical trial of the maternal RSV vaccine, ResVax.126 This global maternal vaccination study of more than 4600 pregnant women failed to meet its primary endpoint of a 41% reduction in medically significant RSV lower respiratory tract infections (LRTI) in infants through their first 90 days of life. However, maternal ResVax immunization did reduce RSV LRTI hospitalizations by 44% through the first 90 days of life. Results were not consistent across regions, with South Africa demonstrating greater disease reduction than in the United States.127 Unfortunately, the study was not powered to determine if this regional difference was due to random variations or if other mechanisms were responsible for improved responses. In August 2019, Novavax released preliminary results from a follow-up study demonstrating a 56% reduction in the incidence of pneumonia in infants born to ResVax immunized mothers through their first year of life.128 Acute and long-term results from the phase III trial demonstrated the safety of maternal RSV immunization.
The discovery and subsequent stabilization of the pre-fusion conformation of RSV F protein (pre-F) has garnered a great deal of excitement for its use as a vaccine antigen due to its ability to elicit high titers of RSV neutralizing antibody.129 Clinically, higher pre-F IgG serum concentrations in full-term infants were associated with lower clinical disease severity scores and possessed greater neutralizing capacity than G protein or post-F IgG.22 Elevated human breast milk concentrations of pre-F IgG were also demonstrated to be a correlate of protection against RSV acute respiratory infections in a cohort of Nepalese mother–infant pairs.23 In an experimental infection of human adults with RSV, elevated titers of pre-challenge RSV-specific nasal IgA were correlated with protection from RSV challenge.25 However, there was no association between breast milk pre-F-specific IgA and protection from acute RSV infection in the Nepalese mother–infant pairs.23
As a result of pre-Fs greater neutralization capacity and correlation with reduced disease severity, it is currently being explored as an antigen in maternal vaccine formulations by GlaxoSmithKline (NCT02753413), Pfizer (NCT03529773), and the NIAID (NCT03049488). The RSV pre-F vaccine candidate being developed by Pfizer is also being evaluated for use in the elderly including in combination with a CpG TLR9 adjuvant. Maternal RSV pre-F vaccine candidates by GlaxoSmithKline and National Institute of Allergy and Infectious Disease (NIAID) are being tested unadjuvanted or with aluminum hydroxide adjuvant.
Active pediatric immunization
The feasibility of direct RSV vaccination of infants has been called into question due to the short time frame (2–3 months) between birth and peak RSV-related hospitalizations.131 Direct vaccination of infants is also complicated by their lower vaccine responses and maternal antibody interference. However, nearly 40% of hospitalizations and 75% of RSV-related outpatient visits occur in children >6 months of age.131 In addition, older children most commonly introduce younger siblings to RSV,132 so a delayed vaccination strategy may still reduce the burden of infant RSV disease while allowing for immunological maturation and a decay of suppression due to maternal antibody titers. Recent detailed analysis of infant RSV antibody specificity following natural infection demonstrated that age-dependent differences in epitope specificity for antigenic sites on RSV F protein exist.67 Encouragingly, infants were able to generate potent neutralizing antibodies.
Live-attenuated and chimeric vaccines
Development of live-attenuated RSV vaccines began in earnest following the tragic failure of the first FI-RSV vaccine trials of the 1960s. As described previously, alum-adjuvanted FI-RSV vaccination primed for ERD upon community-based RSV exposure whereas live RSV vaccines may not prime for ERD. Furthermore, animal studies demonstrated that RSV exposure prior to FI-RSV vaccination appears to abrogate FI-RSV-related ERD.133 Clinical studies of live-attenuated RSV vaccine candidates administered to nearly 400 RSV-naïve infants and children confirmed an absence of ERD after RSV exposure.134 Live-attenuated vaccines are administered intranasally and their immunogenicity is partially dependent on their level of replication within the respiratory tract. Active replication induces broad cellular and humoral responses as well as local mucosal immunity. There is a suggestion they may have effect despite the presence of maternal neutralizing antibody.135 The degree of viral replication of live RSV vaccines is inversely correlated with the level of attenuation.135 This delicate balance of attenuation and immunogenicity is difficult to achieve, with over-attenuation resulting in a lack of immunogenicity and under-attenuation potentially leading to increased adverse events. Most live-attenuated vaccine candidates currently being investigated have a large gene deletion, which reduces the risk of de-attenuation, the most serious concern for live-attenuated vaccines. The basis of attenuation of a number of live-attenuated pediatric vaccine candidates is a deletion within the RSV M2-2 gene. The M2-2 gene regulates the switch from transcription to RNA replication, which makes it an attractive target for attenuation. Deletion of M2-2 increases viral mRNA accumulation within the cell and reduces viral RNA replication. Increased mRNA accumulation is accompanied by an increase in the expression of RSV proteins, including the major antigenic F and G glycoproteins, suggesting that M2-2 deletion mutants may have improved immunogenicity over wild-type virus.136 A second target for reverse genetics is deletion of the non-structural genes 1 or 2 (NS1 and NS2), which are responsible for antagonizing types I and III interferon responses and inhibiting the apoptosis of airway epithelial cells.137–139 Additional modifications to the virus, such as point mutations and amino acid substitutions are incorporated to attenuate the virus further. Currently, there are five live-attenuated RSV vaccines in phase I/II clinical studies that are being developed in partnership with the National Institutes of Health. In September 2019, Meissa Vaccines announced that it would initiate phase I/II clinical trials of its MV-012-968 live-attenuated RSV vaccine candidate.140 Meissa’s vaccine candidate uses reverse genetics and synthetic biology to stabilize antigenic conformations and highly attenuate the candidate vaccine. Pre-clinical studies demonstrated that MV-012-968 exhibited elevated pre-fusion antigen levels, had thermal stability, was highly attenuated, and was protective of RSV challenge in cotton rats.141 There is one chimeric vaccine candidate in phase I testing, rBCG-N-hRSV, which comprises recombinant strains of Mycobacterium bovis bacillus Calmette–Guerin expressing RSV nucleoprotein (N). In pre-clinical studies using mice, rBCG-N-hRSV immunization induced a Th1-dominant response that protected from RSV challenge, with significantly reduced airway inflammatory cell infiltration.142,143 However, testing was performed in adult mice and it remains to be determined if similar Th1-dominant responses will be elicited in infants.
Vector-based vaccines
Vector-based vaccines are used to display various RSV viral proteins to induce immunity. Several vector-based RSV vaccines are currently being evaluated in pediatric populations. Janssen is developing a RSV vaccine candidate in which pre-F is being expressed in the human adenovirus strain 26 (Ad26). The Ad26 vector has been used to display HIV envelope protein, inducing diverse humoral and cellular immune responses.144 GlaxoSmithKline is also pursuing a vector-based RSV vaccine approach. They are using a replication-incompetent chimpanzee adenovirus vector to display the RSV F, N, and M2-1 proteins (ChAd155-RSV). In a phase I study in RSV-immune adults, ChAd155-RSV generated increases in neutralizing antibodies and RSV F-specific interferon γ-secreting T cells,145 suggesting that ChAd155-RSV is immunogenic even in the presence of pre-existing RSV immunity. Phase II testing of ChAd155-RSV in RSV-naïve infants began in April 2019 (Table 1).
Table 1.
Overview of RSV vaccines and antibodies currently under investigation.
| Immunoprophylaxis | |||
|---|---|---|---|
| Antibody/vaccine | Company/institute | Target/modification/vector | Development phase |
| MEDI8897 | AstraZeneca/Sanofi Pasteur | Anti-RSV F monoclonal antibody | Phase II/III |
| MK-1654 | Merck Sharp/Dohme Corp | Broadly neutralizing anti-RSV F site IV antibody | Phase I/II |
| Maternal | |||
| ResVax | Novavax | RSV F nanoparticle vaccine | Phase III |
| GSK3888550A/RSVPreF3 | GlaxoSmithKline | RSV Pre-F subunit vaccine | Phase I |
| RSV preF vaccine | Pfizer | RSV Pre-F subunit vaccine | Phase I/II |
| VRC-RSVRGP084-00-VP | NIAID | RSV Pre-F subunit vaccine | Phase I |
| Live-attenuated/chimeric | |||
| ITV-RSV-ΔG | Intravacc | RSV G deletion | Phase I |
| RSV 6120/ΔNS2/1030s | NIAID | RSV NS2 deletion | Phase II |
| RSV ΔNS2 Δ1313 I1314L | Sanofi/LID/NIAID/NIH | RSV NS2 deletion | Phase I |
| RSV 276 | Sanofi/LID/NIAID/NIH | RSV M2-2 deletion | Phase II |
| MV-012-968 | Meissa Vaccines | Codon deoptimization of NS1/NS2/G, SH deletion, and ablation of secreted form of G | Phase I/II |
| SeV/RSV | SIIPL/St. Jude Hospital | RSV F-expressing SeV carrier | Phase I |
| rBCG-N-hRSV | Pontificia Universidad Catolica de Chile | RSV N-expressing recombinant chimera | Phase I |
| Vector-based | |||
| AdCy7-Fsyn | Weill Cornell Medical College | RSV F-expressing chimpanzee adenoviral vector | Preclinical |
| Ad26.RSV.preF | Janssen | RSV Pre-F-expressing human adenoviral vector | Phase II |
| ChAd155-RSV | GlaxoSmithKline | RSV F/N/M2-1-expressing replication incompetent chimpanzee adenoviral vector | Phase II |
| Subunit | |||
| RSV G protein | Beijing Advaccine Biotechnology | RSV recombinant G protein + low-dose cyclosporine A | Phase I |
LID, Laboratory of Infectious Diseases; NIAID, National Institutes of Allergy and Infectious Diseases; NIH, National Institutes of Health; SIIPL, Serum Institute of India Pvt. Ltd.
Subunit vaccines
Pre-F antigens have potential for use as an infant vaccine. However, in order to avoid the issue of ERD with such antigens it will be imperative to avoid formulating infant pre-F vaccines with Th2-biasing adjuvants such as aluminum salts or squalene adjuvants such as MF59; but instead formulate these vaccines with more balanced Th1/Th2 adjuvants, such as Advax or Advax combined with CpG oligonucleotides. Notably, many adjuvants that work well at inducing Th1 responses in adults may have different effects in infants. For example, compared to their potent effects in adult blood, the TLR agonists, lipopolysaccharide (LPS), Pam3CSK4, flagellin and Poly I:C stimulate weak Th1 cytokine production in cord blood monocytes.146–148 The squalene oil emulsion adjuvant, MF59, while effective in adults, was ineffective at inducing influenza antibody responses when used to immunize 7-day-old mice, even after a second booster dose was administered at an older age.149 Nevertheless, some adjuvants may remain effective even in neonates. A lipidated TLR7/8 adjuvant enhanced antibody responses to a pneumococcal conjugate vaccine when given to 1-day-old macaques.150 Another adjuvant shown to induce balanced Th1 and Th2 responses in infant models is Advax (delta inulin) (reviewed in Honda-Okubo et al.).151 A single-dose of Advax-adjuvanted influenza vaccine given to mouse pups at 7 days of age succeeded in producing protective influenza-specific IgG responses, memory B cells and T cells secreting IFN-γ IL-2, IL-4 and IL-17, with complete protection against influenza infection.152 In contrast, the unadjuvanted influenza vaccine provided no protection to the infant mice, even though the same vaccine was protective in adults.152 Of relevance to the issue of ERD, formulation of inactivated or recombinant Severe Acute Respiratory Syndrome (SARS) coronavirus vaccines with Advax adjuvant alone or co-formulated with a TLR9 active CpG oligonucleotide provided robust protection against SARS and prevented vaccine-associated eosinophilic lung pathology, a problem of alum-adjuvanted SARS vaccines.153 Hence, an optimally protective neonatal RSV vaccine without the risks of VED might be created by combining a pre-F antigen with Advax adjuvant, with such a vaccine currently under study by the authors with promising results.154-155
Summary and conclusion
RSV is the leading cause of morbidity and mortality in children worldwide. Efforts to develop an effective RSV vaccination began shortly after its discovery in the late 1950s. These initial attempts at direct pediatric immunization resulted in severe ERD and the deaths of two children upon natural infection. These tragic results have held back further attempts to develop an infant RSV vaccine. Advances in understanding of RSV now allow refreshed approaches to pediatric RSV vaccination. This includes the possibility of using maternal immunization for short-term passive protection of newborns, or direct infant immunization using pre-F antigen formulated with newer adjuvants such as Advax that elicit a more Th1 balanced immune response. Such advances will benefit from a more complete understanding of infant responses to RSV infections and immunization through the use of age-specific animal models.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Center For Advancing Translation Sciences of the National Institutes of Health (K. M. Eichinger: TL1TR001858); National Institutes of Health (K. M. Eichinger: 1T32AI089443); National Institutes of Health (K. M. Empey: R43AI140941); David and Betty Brenneman Fund (K. M. Empey); Vaxine Pty Ltd (K. M. Empey); and Calder Biosciences (K. M. Empey). Development of Advax adjuvant was supported by National Institutes of Health (NIH) contracts AI061142 and HHSN272200800039C and HHSN272201400053C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of interest: Nikolai Petrovsky is affiliated with Vaxine Pty Ltd, which owns the Advax adjuvant technology. Katherine M. Eichinger, Jessica L. Kosanovich, Madeline Lipp, and Kerry M. Empey have no conflicts of interest to declare.
ORCID iD: Nikolai Petrovsky  https://orcid.org/0000-0002-1580-5245
https://orcid.org/0000-0002-1580-5245
Contributor Information
Katherine M. Eichinger, Department of Pharmacy and Therapeutics, University of Pittsburgh School of Pharmacy, and Clinical and Translational Science Institute, University of Pittsburgh, Pittsburgh, PA, USA
Jessica L. Kosanovich, Department of Pharmacy and Therapeutics, University of Pittsburgh, Pittsburgh, PA, USA
Madeline Lipp, Department of Pharmacy and Therapeutics, University of Pittsburgh School of Pharmacy, University of Pittsburgh, Pittsburgh, PA, USA; Department of Pharmaceutical Sciences, University of Pittsburgh School of Pharmacy, University of Pittsburgh, Pittsburgh, PA, USA.
Kerry M. Empey, Department of Pharmacy and Therapeutics, Department of Pharmaceutical Sciences, School of Medicine and Clinical and Translational Science Institute, University of Pittsburgh School of Pharmacy, University of Pittsburgh, Pittsburgh, PA, USA
Nikolai Petrovsky, College of Medicine and Public Health, Flinders University, Bedford Park, South Australia 5042, Australia and Vaxine Pty Ltd, Warradale, SA 5046, Australia.
References
- 1. Collins PL, Crowe JE. Respiratory Syncytial Virus and Metapneumovirus. In: Knipe DM. (ed.) Fields’ virology, 5th edn. Philadelphia: Wolters Kluwer Health Lippincott Williams & Wilkins, 2007, pp. 1601–1636. [Google Scholar]
- 2. Sande CJ, Cane PA, Nokes DJ. The association between age and the development of respiratory syncytial virus neutralising antibody responses following natural infection in infants. Vaccine 2014; 32: 4726–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shay DK, Holman RC, Roosevelt GE, et al. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979–1997. J Infect Dis 2001; 183: 16–22. [DOI] [PubMed] [Google Scholar]
- 4. Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390: 946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scheltema NM, Gentile A, Lucion F, et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Glob Health 2017; 5: e984–e991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kamal-Bahl S, Doshi J, Campbell J. Economic analyses of respiratory syncytial virus immunoprophylaxis in high-risk infants: a systematic review. Arch Pediatr Adolesc Med 2002; 156: 1034–1041. [DOI] [PubMed] [Google Scholar]
- 7. Empey KM, Peebles RS, Jr, Kolls JK. Pharmacologic advances in the treatment and prevention of respiratory syncytial virus. Clin Infect Dis 2010; 50: 1258–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kapikian AZ, Mitchell RH, Chanock RM, et al. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 1969; 89: 405–421. [DOI] [PubMed] [Google Scholar]
- 9. Chin J, Magoffin RL, Shearer LA, et al. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol 1969; 89: 449–463. [DOI] [PubMed] [Google Scholar]
- 10. Fulginiti VA, Eller JJ, Sieber OF, et al. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol 1969; 89: 435–448. [DOI] [PubMed] [Google Scholar]
- 11. Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89: 422–434. [DOI] [PubMed] [Google Scholar]
- 12. Kollmann TR, Crabtree J, Rein-Weston A, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol 2009; 183: 7150–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Opiela SJ, Koru-Sengul T, Adkins B. Murine neonatal recent thymic emigrants are phenotypically and functionally distinct from adult recent thymic emigrants. Blood 2009; 113: 5635–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hendricks DW, Fink PJ. Recent thymic emigrants are biased against the T-helper type 1 and toward the T-helper type 2 effector lineage. Blood 2011; 117: 1239–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams JV, Weitkamp J-H, Blum DL, et al. The human neonatal B cell response to respiratory syncytial virus uses a biased antibody variable gene repertoire that lacks somatic mutations. Mol Immunol 2009; 47: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Myers HI, Spracklen CN, Ryckman KK, et al. A retrospective study of administration of vaccination for hepatitis B among newborn infants prior to hospital discharge at a midwestern tertiary care center. Vaccine 2015; 33: 2316–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao LH, Liu ZM, Zhao PL, et al. Efficacy of combined hepatitis B immunoglobulin and hepatitis B vaccine in blocking father–infant transmission of hepatitis B viral infection. Genet Mol Res 2015; 14: 4651–4657. [DOI] [PubMed] [Google Scholar]
- 18. Chu HY, Steinhoff MC, Magaret A, et al. Respiratory syncytial virus transplacental antibody transfer and kinetics in mother–infant pairs in Bangladesh. J Infect Dis 2014; 210: 1582–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glezen WP, Paredes A, Allison JE, et al. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr 1981; 98: 708–715. [DOI] [PubMed] [Google Scholar]
- 20. Liang B, Surman S, Amaro-Carambot E, et al. Enhanced neutralizing antibody response induced by respiratory syncytial virus prefusion F protein expressed by a vaccine candidate. J Virol 2015; 89: 9499–9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sande CJ, Mutunga MN, Okiro EA, et al. Kinetics of the neutralizing antibody response to respiratory syncytial virus infections in a birth cohort. J Med Virol 2013; 85: 2020–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Capella C, Chaiwatpongsakorn S, Gorrell E, et al. Prefusion F, postfusion F, G antibodies, and disease severity in infants and young children with acute respiratory syncytial virus infection. J Infect Dis 2017; 216: 1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mazur NI, Horsley N, Englund JA, et al. Breast milk prefusion F IgG as a correlate of protection against respiratory syncytial virus acute respiratory illness. J Infect Dis 2019; 219: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shehata L, Wieland-Alter WF, Maurer DP, et al. Systematic comparison of respiratory syncytial virus-induced memory B cell responses in two anatomical compartments. Nat Commun 2019; 10: 1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Habibi MS, Jozwik A, Makris S, et al. Impaired antibody-mediated protection and defective IgA B-cell memory in experimental infection of adults with respiratory syncytial virus. Am J Respir Crit Care Med 2015; 191: 1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cannon MJ, Openshaw PJ, Askonas BA. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med 1988; 168: 1163–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Graham BS, Bunton LA, Wright PF, et al. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest 1991; 88: 1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hussell T, Baldwin CJ, O’Garra A, et al. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur J Immunol 1997; 27: 3341–3349. [DOI] [PubMed] [Google Scholar]
- 29. Olson MR, Hartwig SM, Varga SM. The number of Respiratory Syncytial Virus (RSV)-specific memory CD8 T cells in the lung is critical for their ability to inhibit RSV vaccine-enhanced pulmonary eosinophilia. J Immunol 2008; 181: 7958–7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ostler T, Davidson W, Ehl S. Virus clearance and immunopathology by CD8+ T cells during infection with respiratory syncytial virus are mediated by IFN-γ. Eur J Immunol 2002; 32: 2117–2123. [DOI] [PubMed] [Google Scholar]
- 31. Lee S, Stokes KL, Currier MG, et al. Vaccine-elicited CD8+ T cells protect against respiratory syncytial virus strain A2-line19F-induced pathogenesis in BALB/c mice. J Virol 2012; 86: 13016–13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim K-H, Lee Y-T, Hwang HS, et al. Alum adjuvant enhances protection against respiratory syncytial virus but exacerbates pulmonary inflammation by modulating multiple innate and adaptive immune cells. PLoS One 2015; 10: e0139916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Knudson CJ, Hartwig SM, Meyerholz DK, et al. RSV vaccine-enhanced disease is orchestrated by the combined actions of distinct CD4 T cell subsets. PLoS Pathog 2015; 11: e1004757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cormier SA, Shrestha B, Saravia J, et al. Limited type I interferons and plasmacytoid dendritic cells during neonatal respiratory syncytial virus infection permit immunopathogenesis upon reinfection. J Virol 2014; 88: 9350–9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee YM, Miyahara N, Takeda K, et al. IFN-gamma production during initial infection determines the outcome of reinfection with respiratory syncytial virus. Am J Respir Crit Care Med 2008; 177: 208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marr N, Wang TI, Kam SH, et al. Attenuation of respiratory syncytial virus-induced and RIG-I-dependent type I IFN responses in human neonates and very young children. J Immunol 2014; 192: 948–957. [DOI] [PubMed] [Google Scholar]
- 37. You D, Becnel D, Wang K, et al. Exposure of neonates to respiratory syncytial virus is critical in determining subsequent airway response in adults. Respir Res 2006; 7: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Empey KM, Orend JG, Peebles RS, Jr, et al. Stimulation of immature lung macrophages with intranasal interferon gamma in a novel neonatal mouse model of respiratory syncytial virus infection. PLoS One 2012; 7: e40499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harker JA, Lee DC, Yamaguchi Y, et al. Delivery of cytokines by recombinant virus in early life alters the immune response to adult lung infection. J Virol 2010; 84: 5294–5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee FE, Walsh EE, Falsey AR, et al. Human infant respiratory syncytial virus (RSV)-specific type 1 and 2 cytokine responses ex vivo during primary RSV infection. J Infect Dis 2007; 195: 1779–1788. [DOI] [PubMed] [Google Scholar]
- 41. Burl S, Townend J, Njie-Jobe J, et al. Age-dependent maturation of Toll-like receptor-mediated cytokine responses in Gambian infants. PLoS One 2011; 6: e18185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lisciandro JG, Prescott SL, Nadal-Sims MG, et al. Ontogeny of Toll-like and NOD-like receptor-mediated innate immune responses in Papua New Guinean infants. PLoS One 2012; 7: e36793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haeberle HA, Takizawa R, Casola A, et al. Respiratory syncytial virus-induced activation of nuclear factor-kappa B in the lung involves alveolar macrophages and Toll-like receptor 4-dependent pathways. J Infect Dis 2002; 186: 1199–1206. [DOI] [PubMed] [Google Scholar]
- 44. Liu P, Jamaluddin M, Li K, et al. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol 2007; 81: 1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Murawski MR, Bowen GN, Cerny AM, et al. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J Virol 2009; 83: 1492–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Caballero MT, Serra ME, Acosta PL, et al. TLR4 genotype and environmental LPS mediate RSV bronchiolitis through Th2 polarization. J Clin Invest 2015; 125: 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Janssen R, Bont L, Siezen CL, et al. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J Infect Dis 2007; 196: 826–834. [DOI] [PubMed] [Google Scholar]
- 48. Eichinger KM, Egaña L, Orend JG, et al. Alveolar macrophages support interferon gamma-mediated viral clearance in RSV-infected neonatal mice. Respir Res 2015; 16: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Everard ML, Swarbrick A, Wrightham M, et al. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child 1994; 71: 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McNamara PS, Ritson P, Selby A, et al. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch Dis Child 2003; 88: 922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marguet C, Bocquel N, Benichou J, et al. Neutrophil but not eosinophil inflammation is related to the severity of a first acute epidemic bronchiolitis in young infants. Pediatr Allergy Immunol 2008; 19: 157–165. [DOI] [PubMed] [Google Scholar]
- 52. Emboriadou M, Hatzistilianou M, Magnisali C, et al. Human neutrophil elastase in RSV bronchiolitis. Ann Clin Lab Sci 2007; 37: 79–84. [PubMed] [Google Scholar]
- 53. Saravia J, You D, Shrestha B, et al. Respiratory syncytial virus disease is mediated by age-variable IL-33. PLoS Pathog 2015; 11: e1005217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vu LD, Siefker D, Jones TL, et al. Elevated levels of type 2 respiratory innate lymphoid cells in human infants with severe RSV bronchiolitis. Am J Respir Crit Care Med 2019; 200: 1414–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lee H-H, Hoeman CM, Hardaway JC, et al. Delayed maturation of an IL-12-producing dendritic cell subset explains the early Th2 bias in neonatal immunity. J Exp Med 2008; 205: 2269–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Malloy AMW, Ruckwardt TJ, Morabito KM, et al. Pulmonary dendritic cell subsets shape the respiratory syncytial virus-specific CD8+ T cell immunodominance hierarchy in neonates. J Immunol 2017; 198: 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ruckwardt TJ, Malloy AMW, Morabito KM, et al. Quantitative and qualitative deficits in neonatal lung-migratory dendritic cells impact the generation of the CD8+ T cell response. PLoS Pathog 2014; 10: e1003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Roman M, Calhoun WJ, Hinton KL, et al. Respiratory syncytial virus infection in infants is associated with predominant Th-2-like response. Am J Respir Crit Care Med 1997; 156: 190–195. [DOI] [PubMed] [Google Scholar]
- 59. Reed JL, Welliver TP, Sims GP, et al. Innate immune signals modulate antiviral and polyreactive antibody responses during severe respiratory syncytial virus infection. J Infect Dis 2009; 199: 1128–1138. [DOI] [PubMed] [Google Scholar]
- 60. Esposito S, Scarselli E, Lelii M, et al. Antibody response to respiratory syncytial virus infection in children <18 months old. Hum Vaccin Immunother 2016; 12: 1700–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Restori KH, Srinivasa BT, Ward BJ, et al. Neonatal immunity, respiratory virus infections, and the development of asthma. Front Immunol 2018; 9: 1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tian C, Kron GK, Dischert KM, et al. Low expression of the interleukin (IL)-4 receptor alpha chain and reduced signalling via the IL-4 receptor complex in human neonatal B cells. Immunology 2006; 119: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jans J, Pettengill M, Kim D, et al. Human newborn B cells mount an interferon-α/β receptor-dependent humoral response to respiratory syncytial virus. J Allergy Clin Immunol 2017; 139: 1997–2000.e4. [DOI] [PubMed] [Google Scholar]
- 64. Pihlgren M, Schallert N, Tougne C, et al. Delayed and deficient establishment of the long-term bone marrow plasma cell pool during early life. Eur J Immunol 2001; 31: 939–946. [DOI] [PubMed] [Google Scholar]
- 65. Basha S, Surendran N, Pichichero M. Immune responses in neonates. Expert Rev Clin Immunol 2014; 10: 1171–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kaul TN, Welliver RC, Wong DT, et al. Secretory antibody response to respiratory syncytial virus infection. Am J Dis Child 1981; 135: 1013–1016. [DOI] [PubMed] [Google Scholar]
- 67. Goodwin E, Gilman MSA, Wrapp D, et al. Infants infected with respiratory syncytial virus generate potent neutralizing antibodies that lack somatic hypermutation. Immunity 2018; 48: 339–349.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Siegrist C-A, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol 2009; 9: 185–194. [DOI] [PubMed] [Google Scholar]
- 69. Kaur K, Chowdhury S, Greenspan NS, et al. Decreased expression of tumor necrosis factor family receptors involved in humoral immune responses in preterm neonates. Blood 2007; 110: 2948–2954. [DOI] [PubMed] [Google Scholar]
- 70. Lipsky PE, Attrep JF, Grammer AC, et al. Analysis of CD40-CD40 ligand interactions in the regulation of human B cell function. Ann N Y Acad Sci 1997; 815: 372–383. [DOI] [PubMed] [Google Scholar]
- 71. Aberle JH, Aberle SW, Dworzak MN, et al. Reduced interferon-gamma expression in peripheral blood mononuclear cells of infants with severe respiratory syncytial virus disease. Am J Respir Crit Care Med 1999; 160: 1263–1268. [DOI] [PubMed] [Google Scholar]
- 72. Bendelja K, Gagro A, Bace A, et al. Predominant type-2 response in infants with respiratory syncytial virus (RSV) infection demonstrated by cytokine flow cytometry. Clin Exp Immunol 2000; 121: 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Geevarghese B, Weinberg A. Cell-mediated immune responses to respiratory syncytial virus infection: magnitude, kinetics, and correlates with morbidity and age. Hum Vaccin Immunother 2014; 10: 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Welliver TP, Garofalo RP, Hosakote Y, et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis 2007; 195: 1126–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. De Weerd W, Twilhaar WN, Kimpen JL. T cell subset analysis in peripheral blood of children with RSV bronchiolitis. Scand J Infect Dis 1998; 30: 77–80. [DOI] [PubMed] [Google Scholar]
- 76. Anderson LJ, Tsou C, Potter C, et al. Cytokine response to respiratory syncytial virus stimulation of human peripheral blood mononuclear cells. J Infect Dis 1994; 170: 1201–1208. [DOI] [PubMed] [Google Scholar]
- 77. Heidema J, Rossen JW, Lukens MV, et al. Dynamics of human respiratory virus-specific CD8+ T cell responses in blood and airways during episodes of common cold. J Immunol 2008; 181: 5551–5559. [DOI] [PubMed] [Google Scholar]
- 78. Tripp RA, Moore D, Barskey A, IV, et al. Peripheral blood mononuclear cells from infants hospitalized because of respiratory syncytial virus infection express T helper-1 and T helper-2 cytokines and CC chemokine messenger RNA. J Infect Dis 2002; 185: 1388–1394. [DOI] [PubMed] [Google Scholar]
- 79. Eichinger KM, Kosanovich JL, Empey KM. Localization of the T-cell response to RSV infection is altered in infant mice. Pediatr Pulmonol 2018; 53: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. de Souza AP, de Freitas DN, Antuntes Fernandes KE, et al. Respiratory syncytial virus induces phosphorylation of mTOR at ser2448 in CD8 T cells from nasal washes of infected infants. Clin Exp Immunol 2016; 183: 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Paton AW, Goldwater PN. Respiratory syncytial virus modulation of adult and neonatal lymphocyte mitogenic responses and the role of interferon-gamma. Microb Pathog 1990; 9: 235–241. [DOI] [PubMed] [Google Scholar]
- 82. Alwan WH, Record FM, Openshaw PJ. CD4+ T cells clear virus but augment disease in mice infected with respiratory syncytial virus. Comparison with the effects of CD8+ T cells. Clin Exp Immunol 1992; 88: 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kolli D, Bataki EL, Spetch L, et al. T lymphocytes contribute to antiviral immunity and pathogenesis in experimental human metapneumovirus infection. J Virol 2008; 82: 8560–8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Connors M, Kulkarni AB, Firestone CY, et al. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. J Virol 1992; 66: 7444–7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tang YW, Graham BS. Anti-IL-4 treatment at immunization modulates cytokine expression, reduces illness, and increases cytotoxic T lymphocyte activity in mice challenged with respiratory syncytial virus. J Clin Invest 1994; 94: 1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Culley FJ, Pollott J, Openshaw PJ. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J Exp Med 2002; 196: 1381–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Durant LR, Makris S, Voorburg CM, et al. Regulatory T cells prevent Th2 immune responses and pulmonary eosinophilia during respiratory syncytial virus infection in mice. J Virol 2013; 87: 10946–10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Krishnamoorthy N, Khare A, Oriss TB, et al. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat Med 2012; 18: 1525–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Christiaansen AF, Syed MA, Ten Eyck PP, et al. Altered Treg and cytokine responses in RSV-infected infants. Pediatr Res 2016; 80: 702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Loebbermann J, Durant L, Thornton H, et al. Defective immunoregulation in RSV vaccine-augmented viral lung disease restored by selective chemoattraction of regulatory T cells. Proc Natl Acad Sci U S A 2013; 110: 2987–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Huang H, Saravia J, You D, et al. Impaired gamma delta T cell-derived IL-17A and inflammasome activation during early respiratory syncytial virus infection in infants. Immunol Cell Biol 2015; 93: 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Stoppelenburg AJ, Salimi V, Hennus M, et al. Local IL-17A potentiates early neutrophil recruitment to the respiratory tract during severe RSV infection. PLoS One 2013; 8: e78461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014; 134: e1474–e1502. [DOI] [PubMed] [Google Scholar]
- 94. MedImmune. SYNAGIS (palivizumab). Gaithersburgh, MD, USA: MedImmune; https://www.synagis.com/ (accessed Dec 2020). [Google Scholar]
- 95. MedImmune. Synagis [package insert]. Gaithersburgh, MD, USA: MedImmune, May 2017. https://www.synagis.com/synagis.pdf accessed 19 Dec 2020. [Google Scholar]
- 96. Meissner HC, Long SS; American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn. Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics 2003; 112: 1447–1452. [DOI] [PubMed] [Google Scholar]
- 97. American Academy of Pediatrics Committee on Infectious Diseases and American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014; 134: 415–420. [DOI] [PubMed] [Google Scholar]
- 98. The IMpact-RSV study group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998; 102: 531–537. [PubMed] [Google Scholar]
- 99. Feltes TF, Cabalka AK, Meissner HC, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 2003; 143: 532–540. [DOI] [PubMed] [Google Scholar]
- 100. Nair H, Verma VR, Theodoratou E, et al. An evaluation of the emerging interventions against Respiratory Syncytial Virus (RSV)-associated acute lower respiratory infections in children. BMC Public Health 2011; 11 (Suppl. 3): S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375: 1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Blanken MO, Rovers MM, Molenaar JM, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013; 368: 1791–1799. [DOI] [PubMed] [Google Scholar]
- 103. Scheltema NM, Nibbelke EE, Pouw J, et al. Respiratory syncytial virus prevention and asthma in healthy preterm infants: a randomised controlled trial. Lancet Respir Med 2018; 6: 257–264. [DOI] [PubMed] [Google Scholar]
- 104. O’Brien KL, Chandran A, Weatherholtz R, et al. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: a phase 3 randomised double-blind placebo-controlled trial. Lancet Infect Dis 2015; 15: 1398–1408. [DOI] [PubMed] [Google Scholar]
- 105. PATH. RSV and mAB trial tracker. Seattle, USA: PATH, 2019. [Google Scholar]
- 106. American Pharmaceutical Review. FDA grants breakthrough therapy designation for potential RSV medicine MEDI8897. San Francisco, CA, USA: American Pharmaceutical Review, 2019. [Google Scholar]
- 107. Clinicaltrials.gov. A Study to evaluate the safety of MEDI8897 for the prevention of medically attended Respiratory Syncytial Virus (RSV) Lower Respiratory Track Infection (LRTI) in high-risk children. Bethesda, MD, USA: NIH US National Library of Medicine, 2019. https://clinicaltrials.gov/ct2/show/NCT03959488 Accessed 19 Dec 2020. [Google Scholar]
- 108. Clinicaltrials.gov. A Study to Evaluate the Safety and Efficacy of MEDI8897 for the Prevention of Medically Attended RSV LRTI in Healthy Preterm Infants. (MEDI8897 Ph2b). Bethesda, MD, USA: NIH US National Library of Medicine, 2019. https://clinicaltrials.gov/ct2/show/NCT02878330 Accessed 19 Dec 2020. [Google Scholar]
- 109. Domachowske JB, Khan AA, Esser MT, et al. Safety, tolerability and pharmacokinetics of MEDI8897, an extended half-life single-dose respiratory syncytial virus prefusion F-targeting monoclonal antibody administered as a single dose to healthy preterm infants. Pediatr Infect Dis J 2018; 37: 886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tang A, Chen Z, Cox KS, et al. A potent broadly neutralizing human RSV antibody targets conserved site IV of the fusion glycoprotein. Nat Commun 2019; 10: 4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Clinicaltrials.gov. Safety, Tolerability, and Pharmacokinetics of MK-1654 in Infants (MK-1654-002). Bethesda, MD, USA: Merck Sharp & Dohme Corp., NIH US National Library of Medicine, 2018. https://clinicaltrials.gov/ct2/show/NCT03524118 Accessed 19 Dec 2020. [Google Scholar]
- 112. Saso A, Kampmann B. Vaccination against respiratory syncytial virus in pregnancy: a suitable tool to combat global infant morbidity and mortality? Lancet Infect Dis 2016; 16: e153–e163. [DOI] [PubMed] [Google Scholar]
- 113. Lamprecht CL, Krause HE, Mufson MA. Role of maternal antibody in pneumonia and bronchiolitis due to respiratory syncytial virus. J Infect Dis 1976; 134: 211–217. [DOI] [PubMed] [Google Scholar]
- 114. Ogilvie MM, Vathenen AS, Radford M, et al. Maternal antibody and respiratory syncytial virus infection in infancy. J Med Virol 1981; 7: 263–271. [DOI] [PubMed] [Google Scholar]
- 115. Stensballe LG, Ravn H, Kristensen K, et al. Respiratory syncytial virus neutralizing antibodies in cord blood, respiratory syncytial virus hospitalization, and recurrent wheeze. J Allergy Clin Immunol 2009; 123: 398–403. [DOI] [PubMed] [Google Scholar]
- 116. Munoz FM, Jamieson DJ. Maternal immunization. Obstet Gynecol 2019; 133: 739–753. [DOI] [PubMed] [Google Scholar]
- 117. Walsh EE, Wang L, Falsey AR, et al. Virus-specific antibody, viral load, and disease severity in respiratory syncytial virus infection. J Infect Dis 2018; 218: 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sharma A, Wendland R, Sung B, et al. Maternal immunization with chimpanzee adenovirus expressing RSV fusion protein protects against neonatal RSV pulmonary infection. Vaccine 2014; 32: 5761–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jones BG, Sealy RE, Surman SL, et al. Sendai virus-based RSV vaccine protects against RSV challenge in an in vivo maternal antibody model. Vaccine 2014; 32: 3264–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Castilow EM, Meyerholz DK, Varga SM. IL-13 is required for eosinophil entry into the lung during respiratory syncytial virus vaccine-enhanced disease. J Immunol 2008; 180: 2376. [DOI] [PubMed] [Google Scholar]
- 121. Kwon Y-M, Hwang HS, Lee JS, et al. Maternal antibodies by passive immunization with formalin inactivated respiratory syncytial virus confer protection without vaccine-enhanced disease. Antiviral Res 2014; 104: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Delgado MF, Coviello S, Monsalvo AC, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med 2009; 15: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Polack FP, Teng MN, Collins PL, et al. A role for immune complexes in enhanced respiratory syncytial virus disease. J Exp Med 2002; 196: 859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Munoz FM, Piedra PA, Glezen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine 2003; 21: 3465–3467. [DOI] [PubMed] [Google Scholar]
- 125. Muňoz FM, Swamy GK, Hickman SP, et al. Safety and immunogenicity of a respiratory syncytial virus Fusion (F) protein nanoparticle vaccine in healthy third-trimester pregnant women and their infants. J Infect Dis 2019; 220: 1802–1815. [DOI] [PubMed] [Google Scholar]
- 126. Madhi SA, Polack FP, Piedra PA, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med 2020; 383: 426–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Jares Baglivo S, Polack FP. The long road to protect infants against severe RSV lower respiratory tract illness. F1000Res 2019; 8: F1000 Faculty Rev-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Amy Speak. New data from Novavax phase 3 PrepareTM trial of ResVaxTM – Data demonstrate significant vaccine efficacy against infant pneumonia through the first year of life. Presented at the 2019 IDSOG Annual Meeting, 8–10 August 2019, Big Sky, Montana, USA. [Google Scholar]
- 129. McLellan JS, Chen M, Leung S, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 2013; 340: 1113–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol 2008; 82: 2040–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360: 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hall CB, Geiman JM, Biggar R, et al. Respiratory syncytial virus infections within families. N Engl J Med 1976; 294: 414–419. [DOI] [PubMed] [Google Scholar]
- 133. Waris ME, Tsou C, Erdman DD, et al. Priming with live respiratory syncytial virus (RSV) prevents the enhanced pulmonary inflammatory response seen after RSV challenge in BALB/c mice immunized with formalin-inactivated RSV. J Virol 1997; 71: 6935–6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wright PF, Karron RA, Belshe RB, et al. The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine 2007; 25: 7372–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Karron RA, Buchholz UJ, Collins PL. Live-attenuated respiratory syncytial virus vaccines. Curr Top Microbiol Immunol 2013; 372: 259–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bermingham A, Collins PL. The M2-2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc Natl Acad Sci U S A 1999; 96: 11259–11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Bitko V, Shulyayeva O, Mazumder B, et al. Nonstructural proteins of respiratory syncytial virus suppress premature apoptosis by an NF-κB-dependent, interferon-independent mechanism and facilitate virus growth. J Virol 2007; 81: 1786–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Bossert B, Marozin S, Conzelmann KK. Nonstructural proteins NS1 and NS2 of bovine respiratory syncytial virus block activation of interferon regulatory factor 3. J Virol 2003; 77: 8661–8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Buchholz UJ, Ward JM, Lamirande EW, et al. Deletion of nonstructural proteins NS1 and NS2 from pneumonia virus of mice attenuates viral replication and reduces pulmonary cytokine expression and disease. J Virol 2009; 83: 1969–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Tansey B. Meissa scores $30M to test vaccine for dangerous respiratory virus. (2019). https://www.managedlab.com/blog/meissa-scores-30m-to-test-vaccine-for-dangerous-respiratory-virus/ (Sept 2019; accessed 17 Dec 2020)
- 141. Stobart CC, Rostad CA, Ke Z, et al. A live RSV vaccine with engineered thermostability is immunogenic in cotton rats despite high attenuation. Nat Commun 2016; 7: 13916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bueno SM, Gonzalez PA, Cautivo KM, et al. Protective T cell immunity against respiratory syncytial virus is efficiently induced by recombinant BCG. Proc Natl Acad Sci U S A 2008; 105: 20822–20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Soto JA, Gálvez NMS, Rivera CA, et al. Recombinant BCG vaccines reduce pneumovirus-caused airway pathology by inducing protective humoral immunity. Front Immunol 2018; 9: 2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Barouch DH, Liu J, Peter L, et al. Characterization of humoral and cellular immune responses elicited by a recombinant adenovirus serotype 26 HIV-1 Env vaccine in healthy adults (IPCAVD 001). J Infect Dis 2013; 207: 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Cicconi P, Jones C, Sarkar E, et al. First-in-human randomized study to assess the safety and immunogenicity of an investigational Respiratory Syncytial Virus (RSV) vaccine based on chimpanzee-adenovirus-155 viral vector-expressing RSV fusion, nucleocapsid, and antitermination viral proteins in healthy adults. Clin Infect Dis 2020; 70: 2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kollmann TR, Levy O, Montgomery RR, et al. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity 2012; 37: 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Sharma AA, Jen R, Kan B, et al. Impaired NLRP3 inflammasome activity during fetal development regulates IL-1β production in human monocytes. Eur J Immunol 2015; 45: 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Corbett NP, Blimkie D, Ho KC, et al. Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS One 2010; 5: e15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mastelic Gavillet B, Eberhardt CS, Auderset F, et al. MF59 mediates its B cell adjuvanticity by promoting T follicular helper cells and thus germinal center responses in adult and early life. J Immunol 2015; 194: 4836–4845. [DOI] [PubMed] [Google Scholar]
- 150. Dowling DJ, van Haren SD, Scheid A, et al. TLR7/8 adjuvant overcomes newborn hyporesponsiveness to pneumococcal conjugate vaccine at birth. JCI Insight 2017; 2: e91020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Petrovsky N, Cooper PD. Advax, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine 2015; 33: 5920–5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Honda-Okubo Y, Ong CH, Petrovsky N. Advax delta inulin adjuvant overcomes immune immaturity in neonatal mice thereby allowing single-dose influenza vaccine protection. Vaccine 2015; 33: 4892–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Honda-Okubo Y, Barnard D, Ong CH, et al. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J Virol 2014; 89: 2995–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Eichinger KM, Kosanovich JL, Gidwani SV, et al. Prefusion RSV F Immunization Elicits Th2-Mediated Lung Pathology in Mice When Formulated With a Th2 (but Not a Th1/Th2-Balanced) Adjuvant Despite Complete Viral Protection. Front Immunol 2020; 11:1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Eichinger KM, Kosanovich JL, Lipp MA, et al. Maternal immunization with adjuvanted RSV prefusion F protein effectively protects offspring from RSV challenge and alters innate and T cell immunity. Vaccine 2020; 38(50):7885–7891. [DOI] [PMC free article] [PubMed] [Google Scholar]