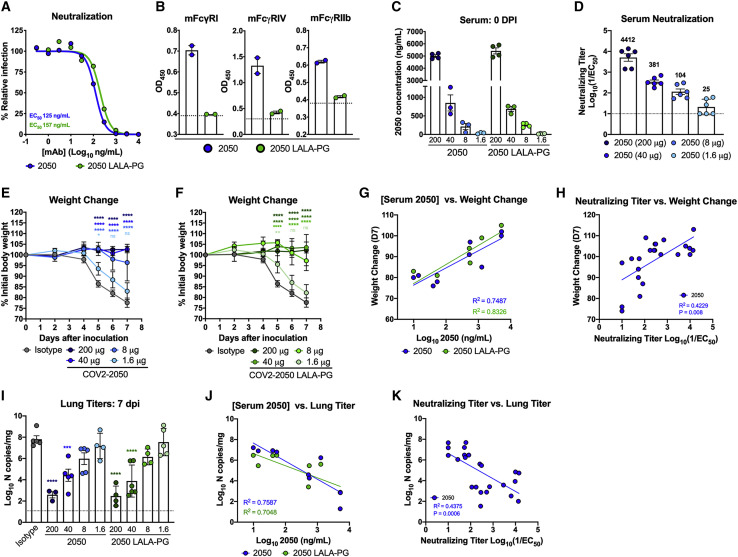
Figure 1.
Neutralizing activity is sufficient for prophylactic efficacy of mAbs against SARS-CoV-2 infection in K18-hACE2 mice
(A) MAbs (COV2-2050 and COV2-2050 LALA-PG) were incubated with 102 focus-forming units (FFU) of SARS-CoV-2 for 1 h followed by addition to Vero E6 cells. Wells containing mAb were compared to wells without mAb to determine relative infection. One experiment with the mean of 2 technical replicates is shown.
(B) Binding of COV2-2050 or COV2-2050 LALA-PG to murine FcγRI, FcγRIV, or FcγRIIb by ELISA (two experiments). The dotted line indicates the limit of detection (LOD), as determined by background binding to a negative control.
(C–K) Eight-week-old female and male K18-hACE2 mice received 200, 40, 8, or 1.6 μg of COV2-2050 or COV2-2050 LALA-PG by intraperitoneal injection 1 day prior to intranasal inoculation with 103 PFU of SARS-CoV-2.
(C) Serum concentrations (ng/mL) of COV2-2050 or COV2-2050 LALA-PG at the time of challenge (0 dpi) (mean ± SEM; n = 3–4, 2 experiments).
(D) Neutralizing titers in serum of indicated groups at 0 dpi as measured by FRNT (mean ± SEM; n = 6, 2 experiments).
(E and F) Weight change following COV2-2050 (E) or COV2-2050 LALA-PG (F) administration (mean ± SEM; n = 4–6, 2 experiments).
(G) Correlation analyses comparing COV2-2050 or COV2-2050 LALA-PG serum concentrations (day 0 [D0]) against weight change (D+7) (n = 4–6, 2 experiments).
(H) Correlation analyses comparing COV2-2050 neutralizing titers in serum (D0) against weight change (D+7) (n = 6–8, 3 experiments).
(I) Viral RNA levels at 7 dpi in the lung (n = 4–6, 2 experiments).
(J) Correlation analyses comparing COV2-2050 or COV2-2050 LALA-PG serum concentrations (D0) against lung viral titer (D+7) (n = 4–6, 2 experiments).
(K) Correlation analyses comparing serum neutralizing titers (D0) against lung viral titer (D7) (n = 6–8, 2 experiments).
(E and F) Two-way ANOVA with Sidak’s post-test: ns not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001; comparison to the isotype control mAb-treated group. (G, H, J, and K) Pearson’s correlations: (G) COV2-2050, p = 0.0026; COV2-2050 LALA-PG, p = 0.0006; (H) COV2-2050, p = 0.0008; (J) COV2-2050, p = 0.0022; COV2-2050 LALA-PG, p = 0.0095; and (K) COV2-2050, p = 0.0006. (I) One-way ANOVA with Turkey’s post-test: ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, comparison to the isotype control mAb-treated group.