Figure 6.
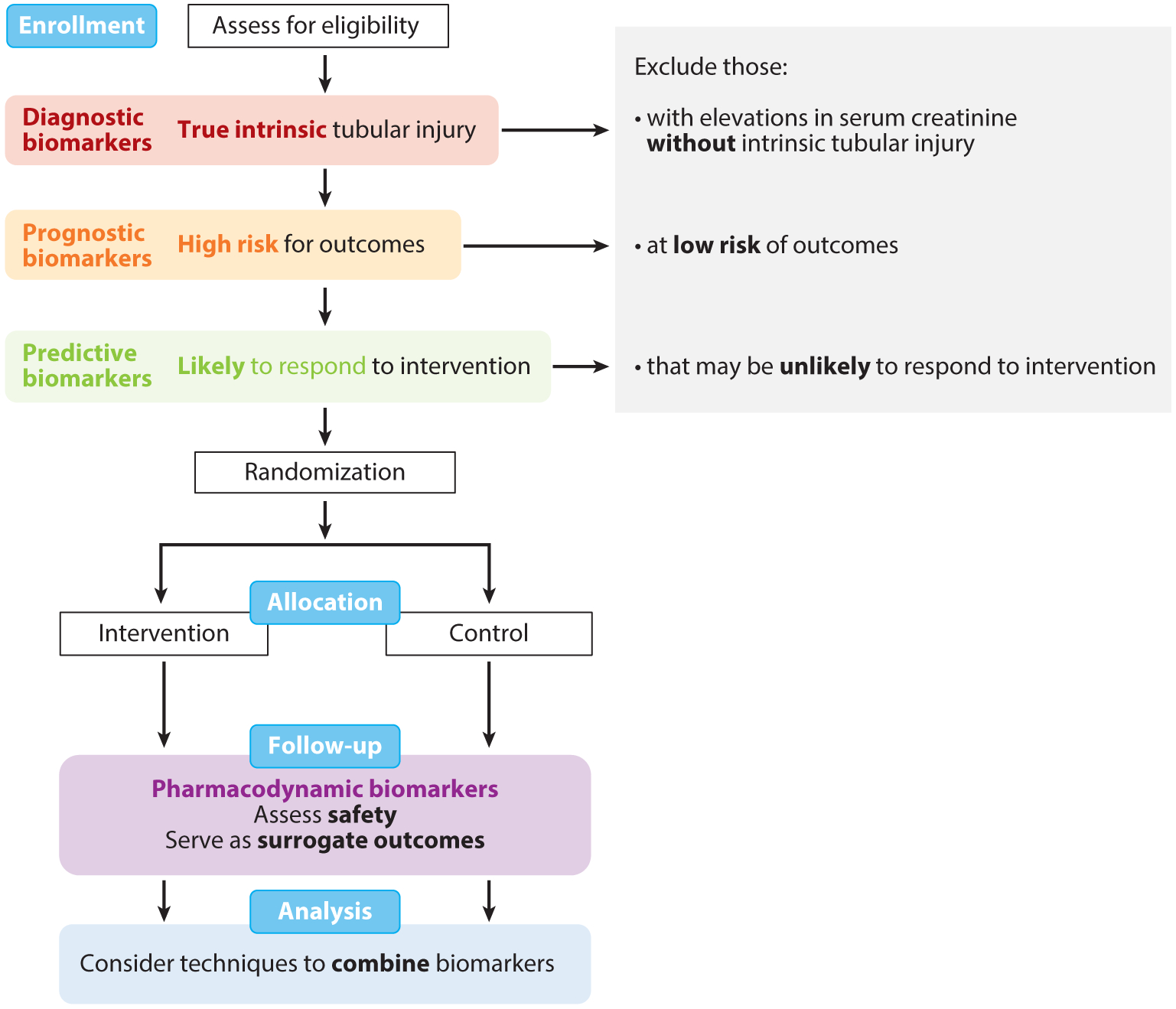
Application of kidney biomarkers in clinical trials. Biomarkers of tubular health may be applied in various stages of clinical trials. At enrollment, diagnostic biomarkers (red) can be used to identify participants with true intrinsic tubular injury; prognostic biomarkers (orange) can be used to identify participants at high risk for outcomes; and predictive biomarkers (green) can be used to identify participants likely to respond to an intervention of interest. By using these biomarkers to assess eligibility and exclude individuals who may not be truly suitable for the study despite an elevation in serum creatinine, investigators may be able to conduct more efficient, cost-effective trials. In the follow-up phase of trials, pharmacodynamics biomarkers (purple) may be used to assess safety of interventions and serve as surrogate outcomes for kidney damage. With the wide breath of biomarkers now available, biomarkers may be combined with novel statistical techniques in the analysis phase. Figure adapted with permission from Reference 140.
