Abstract
Mucopolysaccharidoses (MPS) are inherited metabolic disorders that result from a deficiency of lysosomal enzymes required for the catabolism of glycosaminoglycans (GAG). Lysosomal GAG accumulation results in cell and organ dysfunction. This study characterizes the phenotype and genotype of MPS VI in a Great Dane puppy with clinical signs of stunted growth, facial dysmorphia, skeletal deformities, corneal opacities, and increased respiratory sounds. Clinical and pathological evaluations, urine GAG analyses, lysosomal enzyme assays and ARSB sequencing were performed. The urine mucopolysaccharide spot test was strongly positive predominantly due to the accumulation of dermatan sulfate. Enzyme assays in leukocytes and tissues indicated a deficiency of arylsulphatase B (ARSB) activity. Histologic examinations revealed cytoplasmic vacuoles in many tissues. Analysis of the exonic ARSB DNA sequences from the affected puppy compared to the published canine genome sequence revealed a homozygous nonsense mutation (c.295C>T) in exon 1, replacing glutamine with a premature stop codon (p.Gln99*) predicting no enzyme synthesis. A polymerase chain reaction based restriction fragment length polymorphism test was established to assist with the clinical diagnosis and breeding of Great Danes. This genotyping test revealed that the clinically healthy parents and some other relatives of the puppy were heterozygous for the mutant allele, but all 200 clinically healthy dogs screened including 15 Great Danes were homozygous for the normal allele. This ARSB mutation is the fourth identified genetic variant causing canine MPS VI. MPS VI is the first lysosomal storage disorder described in Great Danes but does not appear to be wide-spread in this breed.
Keywords: arylsulphatase B, dogs, genetic diseases, hereditary disease, lysosomal storage disease, skeletal deformity
Mucopolysaccharidoses (MPS) are a group of inherited lysosomal storage disorders in which a specific lysosomal enzyme required for the step-wise catabolism of glycosaminoglycans (GAG, originally called mucopolysaccharides) is deficient or dysfunctional. Cartilage, bone, skin, blood vessels, tendon and cornea require GAG for maintaining the structure and function of these tissues. The physiological concentration of GAG is controlled by enzymatically regulated metabolic pathways. If the activity of any catabolic lysosomal enzyme is severely reduced, lysosomal GAG accumulation occurs in tissues, causing progressive and irreversible cellular damage that affects growth, morphology, mobility, organ function, and sometimes neurological development.21 To complete degradation of GAG, 11 different lysosomal enzymes are required. Deficiency of any one of them will result in a specific type of MPS.16
MPS type VI (Maroteaux-Lamy Syndrome, Online Mendelian Inheritance in Man #253200) is an autosomal recessive lysosomal storage disorder caused by a deficiency of N-acetylgalactosamine-4-sulfatase (also called arylsulphatase B [ARSB], EC 3.1.6.12). This enzyme plays a critical role in the catabolism of dermatan sulfate. The typical clinical features of MPS VI include progressive abnormal cartilage and bone development leading to short stature, dysostosis multiplex, degenerative joint disease, growth retardation, physical disability, facial malformations, and corneal clouding. Despite GAG storage being systemic and commonly involving the central nervous system, patients with MPS VI usually do not have primary neurological signs but rather severe skeletal and ocular signs.27
Currently, over 150 ARSB gene mutations have been documented in humans with MPS VI (The Human Gene Mutation Database, http://www.hgmd.org) despite representing an orphan disorder. Furthermore, MPS VI has been described in several animal species.14,15,17,18,22,23,32
Canine MPS I, III, VI, and VII have been previously described and share close similarities in clinical disease presentation with other species. With the exception of MPS IIIB (Sanfilippo syndrome B) which is predominantly an adult onset neuropathy, the other MPS disorders largely affected the joints, bones and eyes. The clinical manifestations include growth retardation, facial dysmorphia, pectus excavatum, hip and other joint laxities, umbilical hernia, corneal clouding, hepatomegaly, and cardiovascular dysfunction. These dogs die or are humanly euthanized before they reach adulthood. Dogs with MPS serve as naturally occurring models to understand the disease and assess therapeutics for humans and animals.14
Here we report the clinicopathological, histopathological, biochemical, and molecular genetic characteristics of a Great Dane puppy with MPS VI and its family.
Materials and Methods
Animal, Samples Collection and Preparation
A 4-month-old male Great Dane puppy (proband) with the clinical signs of a possible inborn error of metabolism was referred to the Pediatrics, Genetics and Reproduction Clinic and PennGen Laboratory Services at the School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA for diagnosis and management. Urine, serum and ethylenediaminetetraacetic acid (EDTA)–anticoagulated blood samples were collected. The proband was euthanized at 6 months of age and representative tissues (no spine and brain) were obtained by the Quality Pet Care Clinic in Monticello, NY for histopathological and biochemical analyses. Furthermore, EDTA-anticoagulated blood or buccal swab samples from the proband’s relatives were obtained, and archived DNA samples from clinically healthy dogs, including Great Danes, were genotyped. The investigations were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Leukocytes were purified from fresh EDTA-anticoagulated blood using a dextran density gradient separation technique.12 Fresh liver and spleen samples (20 mg) were homogenized in 1.0 ml of 0.9% saline with 0.2% Triton X-100, and the extracts of the lysates were separated by centrifugation. Protein concentrations were determined by the Bradford-dye protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Genomic DNA was extracted from EDTA blood and buccal swabs using the Qiagen DNA purification kit (Qiagen, Valencia, CA, USA). These samples were stored at −80°C for further study.
Representative tissues were fixed in 10% neutral buffered formalin, routinely processed, embedded in paraffin, and sectioned at 5 μm. Histologic sections were stained with hematoxylin and eosin (HE) for routine examination, periodic acid–Schiff (PAS) to detect polysaccharides including GAG, and Toluidine blue to specifically detect GAG storage, as previously described.26
Urinary GAG Analysis
The Toluidine blue mucopolysaccharide spot test2 was performed on the urine from the proband and a normal juvenile dog. Urine (5 μl) was applied on filter paper (Whatman™ 3MM) and stained with 0.2% Toluidine blue-O for 45 sec and then destained with 0.6% acetic acid. The occurrence of a purple (metachromatic) spot indicated the presence of GAG in urine. The type of GAG was analyzed by thin layer chromatography (Biocontrol, Ingelheim, Germany).
Lysosomal Enzyme Assays
Using 4-methylumbelliferyl (4-MU) based substrates, the activities of 3 lysosomal enzymes, ARSB (for MPS VI), α-L-iduronidase (EC 3.2.1.76 for MPS I), and β-D-glucuronidase (EC 3.2.1.31 for MPS VII) were examined in leukocytes, liver and spleen of the proband and a healthy control juvenile dog. Activities of α-L-iduronidase and β-D-glucuronidase were assayed by previously established fluorescence methods.11 The ARSB activity was measured by a slightly modified protocol:7 Ten μl of tissue extract containing 10 μg protein was incubated with 5.0 mM fluorescent substrate 4-MU sulfate potassium salt (4-MUS, Glycosynth, Warrington, United Kingdom) prepared with acetate buffer (50 mM, pH 5.6) containing 3.0 mM lead acetate and 0.3 mM silver nitrate (both from Sigma-Aldrich, St. Louis, MO, USA) in a final volume of 200 μL for 2 hours at 37°C. The reaction was terminated with 2 ml of stopping buffer (0.32 M glycine, 0.2 M sodium carbonate and 1 mM EDTA, pH 10). The substrate fluorescence signal was measured with a fluorometer (VersaFluor™, Bio-Rad Laboratories, Hercules, CA, USA) at an excitation and emission wavelength of 365 nm and 450 nm, respectively. The results of the specific enzyme activities were calculated from a standard curve of 4-MU and expressed as nM of 4-MU released per mg of protein per hour.
ARSB Gene Sequencing
The 8 exons of the ARSB gene were sequenced from genomic DNA of the proband, its sire and a unrelated healthy dog using previously established hot-start polymerase chain reaction (PCR) and automated Sanger sequencing techniques.28 The PCR primers surrounding the exons and conditions are listed in Supplemental Table 1. The sequencing results were analyzed using Lasergene software (DNASTAR Inc., Madison, WI, USA) and compared to the published canine genome sequence (NCBI Genbank, Gene ID: 610364, RefSeq# NM_001048133.1, http://www.ncbi.nlm.gov) and Ensembl: Transcript ID ENSCAFT00000014585.3, CanFam3.1 canine reference genome assembly ( http://www.ensembl.org), as well as to the sequencing results of the sire and the control dog.
Genotyping Test
Once the ARSB mutation was discovered, a genotyping test using a restriction fragment length polymorphism (RFLP) allele specific analysis was developed for screening EDTA blood and buccal swab samples of the proband, its family members, and other samples. The DNA fragment containing the mutation was amplified using the PCR primers listed in Supplemental Table 1. The BmtI restriction endonuclease recognizes the bases 293-298 of ARSB exon 1 (5’…GCTAG^C…3’), which is present in the mutant but not in the wild-type/normal allele. Thus, the uncut fragment consists of 318 base pairs (bp), while the cut fragments are 278 and 40 bp long. Ten μl of the digestion mixture containing BmtI (10,000 units/ml), 10X NEBuffer 3.1 (both were from New England Biolabs, Inc., Ipswich, MA, USA), and dH2O at a ratio of 0.5: 2.0: 7.5 was mixed with 10 μl of the PCR product and digested at 37°C for 2 hours. The PCR fragments were separated on a 2% agarose gel by electrophoresis and stained with ethidium bromide for visualization.
Results
Clinical Findings
A 4-month-old male Great Dane puppy was presented to the primary care clinician for its 3rd booster vaccinations. At the time, he was also noted to have hind limb splaying with progressive weakness over the previous 2 weeks. The puppy was purchased from a breeder but no pedigree information was made available. The breeder did not recall any similar manifestations in the remainder of the litter or any of the puppies previously produced in the kennel.
The puppy was then referred to a specialist clinic where it appeared disproportionally stunted and had a body condition score of 2/9 at a weight of 16 kg, normal temperature, heart rate, and respiratory rate with slightly increased lung and upper airway sounds. Other abnormalities noted included firm swellings of the paws, valgus deformities of both pelvic limbs, various deformities of both forelimbs, and prominent pelvic bones with bilateral swelling perianally, likely representing pressure point seromas. It was ambulatory with normal cranial nerve responses and conscious proprioception, but had severe neck pain. Results of an instrumental complete blood count, chemistry screen and urinalysis were within normal reference ranges for the patient’s age.
Radiographs revealed severe generalized skeletal abnormalities including irregularity of the vertebral endplates of the entire spine, mild soft tissue swelling of the elbows, bilateral cranial bowing of antebrachii, bowing of metacarpal bones, soft tissue swelling within the metacarpophalangeal joints, abnormal angulation of proximal femurs, generally decreased bone density of hind limbs, with bilaterally increased intramedullary density of the mid tibial diaphysis and irregular margins of the femoral condyles. Additionally, increased opacities in the larynx region were noted, likely responsible for the loud upper respiratory sounds.
Results of synovial fluid analyses from several joint taps showed turbid, light red to light orange fluid with a specific gravity of 1.017 and containing 20,000-50,000 red blood cells/μl,120-150 mononuclear cells/μl including macrophages, synoviocytes, and small mature lymphocytes, but neither neoplastic cells nor infectious organisms were noted. Bacterial culture of the synovial fluid was negative.
The proband (Fig. 1) was referred to the Pediatrics, Genetics and Reproduction clinic at the School of Veterinary Medicine of the University of Pennsylvania. In addition to the previously noted clinicopathological and imaging manifestations, mild corneal opacities, facial dysmorphia and muzzle sloping, with a prominence of the rostral ventral mandible, prognathism, and clear serous nasal discharge and laryngeal sensitivity were observed. Spinal palpation was unremarkable, but pain was elicited on dorsal neck extension.
Figure 1.
Mucopolysaccharidosis type VI Great Dane puppy. 4 months of age. The puppy has stunted growth and swollen joints and paws.
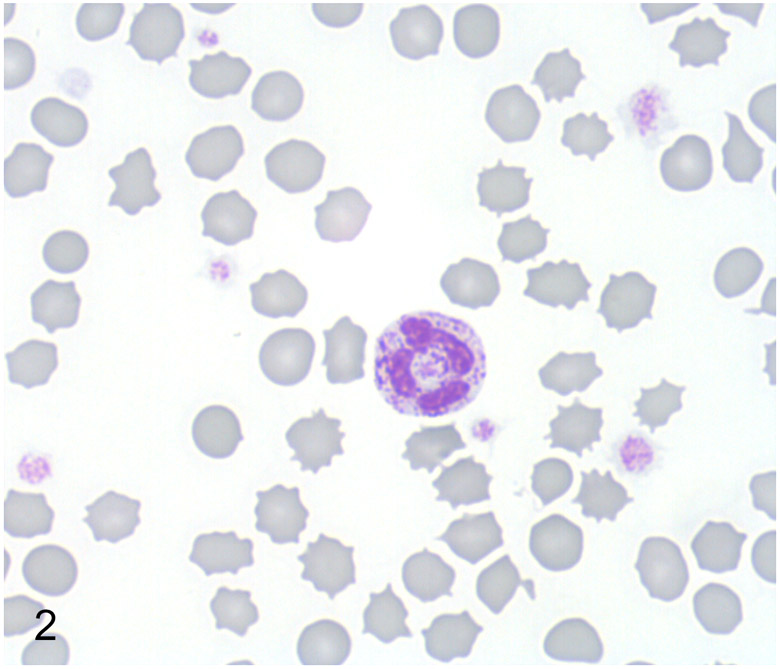
Routine automated complete blood count, chemistry screen, and urinalysis of the proband were again within normal reference ranges. In a blood smear stained with Wright-Giemsa granulocytes, lymphocytes, and monocytes contained moderate to abundant, fine to coarse dark purple cytoplasmic granules, suggestive of a storage disease (Fig. 2).
Figure 2.
Mucopolysaccharidosis type VI, peripheral blood, Great Dane puppy. A neutrophil with many coarse metachromatic granules. Wright-Giemsa.
Histopathologic Findings
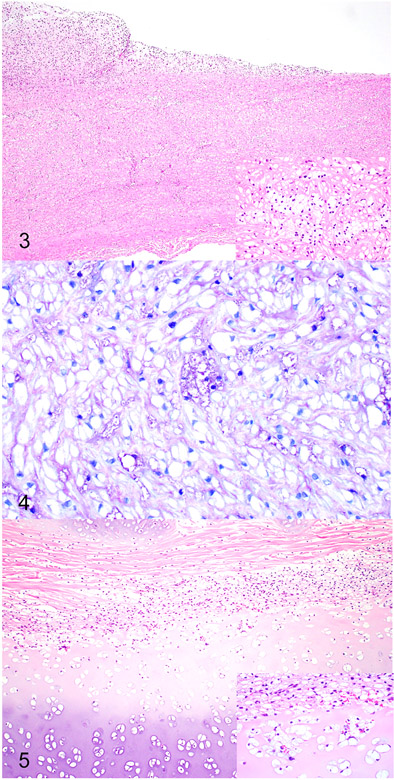
As previously described in cases of canine MPS VI, cytoplasmic vacuoles (i.e. storage material) were observed within macrophages, fibroblasts, and chondrocytes throughout the body.18,22,23 The cytoplasmic vacuolation was most notable within the corneal and uveal stroma, heart valves, Kupffer cells of liver, macrophages of spleen, laryngeal cartilage, tracheal cartilage, and cartilage of the costochondral junction of the rib. The vacuoles stained moderately to strongly positive with a PAS stain and metachromatically with the Toluidine blue stain. Along with the smooth muscle and fibroblast storage material, the aorta also exhibited intimal thickening due to proliferating fibroblasts and chondroid metaplasia (Fig. 3). The chondrocytes within this metaplastic focus contained similar cytoplasmic vacuoles (Fig. 4). The trachea (Fig. 5) and ribs contained severe cytoplasmic vacuolation of chondrocytes and fibroblasts with secondary chondroid degeneration and malformation.
Figures 3-4.
Mucopolysaccharidosis type VI, aorta, Great Dane puppy. Figure 3. The wall of the aorta is thickened and the intima is expanded by proliferating fibroblasts forming plaque-like lesion. Inset. The smooth muscle myocytes within the wall of the aorta contain cytoplasmic vacuoles. Hematoxylin and eosin. Figure 4. The vacuoles within the smooth muscle myocytes are mostly clear but occasionally stain metachromatically, consistent with accumulation of glycosaminoglycan. Toluidine blue.
Figure 5. Mucopolysaccharidosis type VI, tracheal cartilage, Great Dane puppy. The tracheal cartilage is thickened with irregular chondrones and surrounded by proliferating fibroblasts. Inset. The chondrocytes and fibroblasts within the wall of the trachea contain cytoplasmic vacuoles. Hematoxylin and eosin.
Biochemical Analyses
Urine GAG Analysis
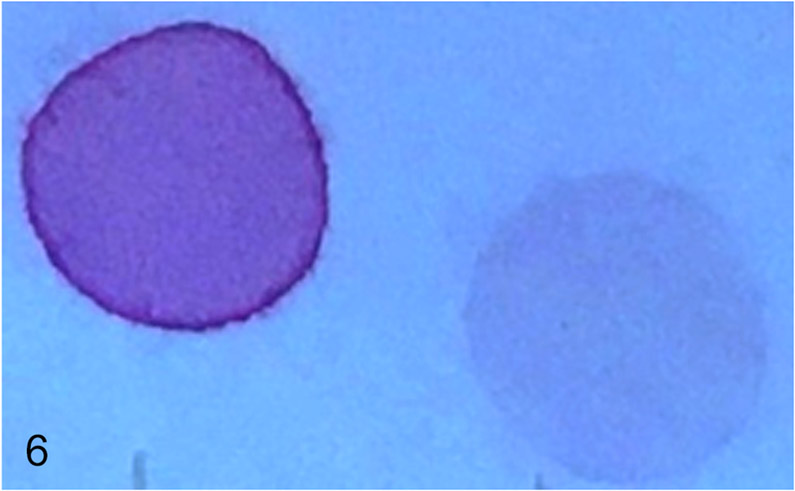
The mucopolysaccharide spot test on urine revealed a strongly positive result (Fig. 6) and chromatographic GAG separation indicated a large amount of dermatan sulfate (date not shown).
Figure 6.
Strongly positive urinary mucopolysaccharide spot test of the suspected MPS affected puppy (left) was compared to a negative result from a normal juvenile control dog (right).
Lysosomal Enzyme Assays
The proband’s ARSB activities in leukocytes, liver and spleen were severely reduced compared to the control (<5% of control). In contrast, the enzymatic activities of α-L-iduronidase and β-D-glucuronidase were in the normal ranges (Table 1).
Table 1.
Lysosomal enzyme activities in leukocytes, liver and spleen of the MPS VI affected dog (Proband), one clinically healthy dog (Control), and normal reference range (Ref.).
| Tissue | Arylsulphatase B (MPS VI)a | α-L-Iduronidase (MPS I) | β-D-Glucuronidase (MPS VII) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Proband | Control | Ref.b | Proband | Control | Ref. | Proband | Control | Ref. | |
| Leukocytes | 1.9c | 40.5 | 45.0±14.4 | 21.2 | 19.3 | 18.7±3.9 | 281.9 | 304.1 | 314.5±88.6 |
| Liver | 1.1 | 23.3 | 26.3±4.5 | 28.8 | 27.7 | 30.6±10.2 | 301.2 | 310.2 | 342.4±90.2 |
| Spleen | 1.4 | 42.1 | 45.0±18.4 | 27.1 | 24.6 | 22.2±9.0 | 408.2 | 426.5 | 400.0±105.3 |
Disease corresponds to the specific dysfunction of the enzyme tested
Reference ranges are based on 10 clinically healthy dogs, and presented as mean ± standard deviation
Enzyme activity: nMol/mg protein/hour
Gene Sequence Analysis
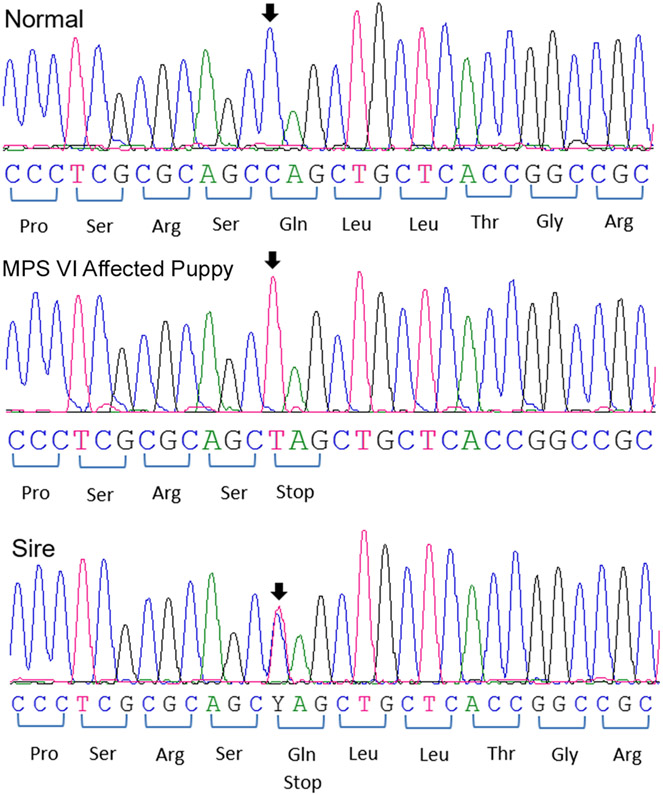
The canine ARSB is located on canine chromosome 3 and consists of 8 exons with the transcript length of 5,785 bp coding for 535 amino acids. The genomic DNA of the proband was examined for the mutations documented in other 3 dog breeds,1,18 but was found to be homozygous normal for each of them. Exonic sequencing of the proband’s DNA revealed a single homozygous nonsense mutation at position 295 in exon 1of ARSB, where a cytosine was exchanged by a thymine (c.295C>T), resulting in the replacement of glutamine (NCBI RefSeq#NP_001041598.1) with a premature stop codon at amino acid position 99 (p.Gln99*). Sequence analyses of DNA showed that the clinically healthy sire was heterozygous for this variant, while an unrelated dog was homozygous normal (Fig. 7).
Figure 7.
DNA sequences of a region of exon 1 of ARSB from a normal dog, the Great Dane puppy with MPS VI, and its sire.
Comparison of the sequencing results also revealed 2 synonymous silent variants in ARSB. One is an exchange of a thymine for a cytosine (c.771T>C) in exon 4. This variant was detected in the sequence (both heterozygous) of the sire and the clinically healthy dog but not the proband. Another variant was the replacement of an adenine with a guanine in exon 8 (c.1599A>G) which was observed in the proband, its sire and the control dog. Both of the variants had been previously documented (variants rs23569849 and rs852721558, respectively, Ensembl CanFam3.1 canine reference genome assembly, Gene ID: ENSCAFG00000009168, http://www.ensembl.org). No other DNA sequence differences were observed in the coding regions between proband and the published ARSB genomic sequence and sequences obtained from the sire and normal control.
Genotyping Test Results of the Great Dane Family and Other Dogs
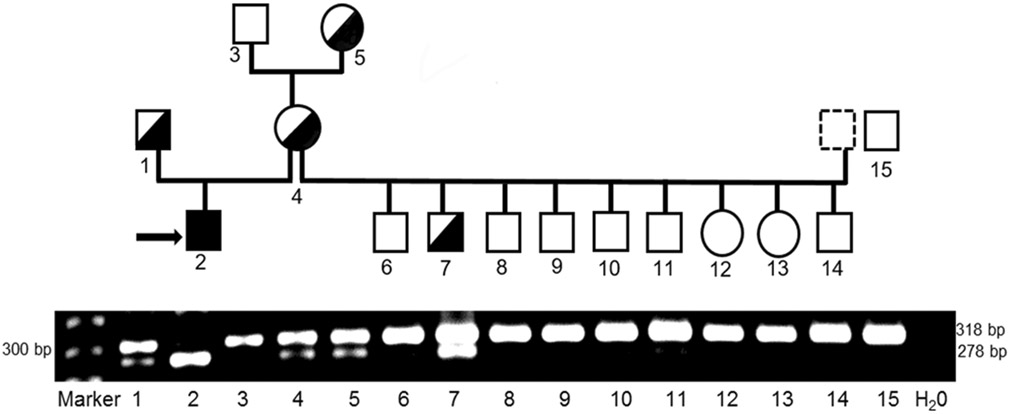
The PCR-RFLP-based genotyping test, which utilizes the BmtI restriction enzyme, cuts the mutant but not normal/wild-type allele thereby clearly differentiating the 2 alleles by size on a gel. The PCR-RFLP results showed that the proband was homozygous for the C>T mutation, while its clinically healthy sire, dam and grand-dam on the maternal side were heterozygous for the normal/wild-type and mutant alleles. In addition, one newborn puppy from a litter of 9 puppies, bred by the proband’s dam with a different sire, was also heterozygous while the others were homozygous normal. All PCR-RFLP results of heterozygous individuals by PCR-RFLP were confirmed by DNA sequencing showing a double peak of C and T on visual examination at position 295. All others, including 15 unrelated healthy Great Dane, were homozygous for the wild-type allele. The gel and corresponding family pedigree illustrates that the mode of inheritance of MPS VI in Great Danes follows an autosomal recessive pattern (Fig. 8).
Figure 8.
Allele-specific genotyping results of Great Dane family with MPS VI by PCR-RFLP.
The intact 318 bp PCR fragment indicates the normal/wild-type allele. The c.295C>T nonsense mutant allele creates a BmtI restriction site, resulting in product cleavage into 278 bp and 40 bp fragments (the 40 bp fragment is too small to be visualized). A 278 bp fragment (homozygous for the C>T mutation allele) corresponds to the MPS VI affected puppy (#2, indicated with an arrow) while the sire (#1), dam (#4), grand-dam (#5) on the maternal side and another puppy (#7) show both the 318 and 278 fragments, indicative of heterozygosity. Others, including an unrelated normal Great Dane (#15), only display the 318 bp fragment (homozygous normal). The numbers labeled on the gel correspond to those in the pedigree. The family pedigree with associated genotypes indicates an autosomal recessive mode of inheritance for MPS VI in Great Dane dogs. Squares and circles represent males and females, respectively. Open (white) and filled (black) symbols designate homozygous normal and mutant, respectively. Half-filled symbols represent heterozygosity.
Discussion
The storage disorder MPS VI has been described in both human and animals including rats,32 cats [Online Mendelian Inheritance in Animals # 000666-9685],15,17 and dogs [# 000666-9615].1,14,18,22,23 Canine MPS VI was reported in the Miniature Pinscher,1,22 Miniature Schnauzer,1,23 Toy Poodle,18 Welsh Corgi,14 and Chesapeake Bay Retriever.14 Molecular studies of canine MPS VI have, thus far, revealed 2 deletions and 1 missense mutation in ARSB.1,18 Furthermore, dogs and cats with MPS VI have been used as large animal models to study the pathophysiology and therapeutic safety and efficacy of novel therapies which include enzyme replacement therapy, bone marrow transplantation, and gene therapy.5,8,24,29
In this study, the Great Dane puppy had early onset of stunted growth, skeletal deformities, corneal opacities and abdominal respiratory effort, along with cytoplasmic granules within blood leukocytes. Histologically, cytoplasmic vacuoles consistent with storage material were observed in the fibroblasts, chrondrocytes, and macrophages throughout the body. These findings, and a strongly positive urine mucopolysaccharide spot test, led to the strong suspicion of a MPS.
The extreme GAG excretion showed by the simple mucopolysaccharide spot test of the proband’s urine was found to be due to excessive dermatan sulfate accumulation. The predominant storage material dermatan sulfate is a polymer with a disaccharide repeating sequence of [IdoA-GalNAc-4S]n which contains a sulfate group that is cleaved by ARSB during the degradation process. Although chondroitin sulfate, another major GAG, is also a natural substrate for ARSB, chondroitin sulfate accumulation is not commonly observed in MPS VI patients due to its catabolism through an alternate pathway. During chondroitin sulfate degradation, the polymer chain is first clipped by the enzymes hyaluronidase and β-glucuronidase resulting in the production of oligosaccharide fragments which are not recognized as chondroitin sulfate storage and therefore, not degraded by ARSB.27 Because of this alternate chondroitin sulfate catabolic pathway, cellular accumulation of dermatan sulfate is the most distinct biochemical maker for ARSB dysfunction, and is indicative of MPS VI.
The diagnosis of MPS VI is generally accomplished by determining a lack of ARSB activity unless the molecular defect has already been determined for the breed. Body tissues and leukocytes contain other kinds of sulfatases than ARSB, such as arylsulfatase A (ARSA). With similar specificity for artificial substrates (4-MUS) and a similarly acidic optimal pH, ARSA may affect the ARSB assay. Chang et al7 reported that ARSA but not ARSB was sensitive to the cationic inhibitors such as Pb2+ and Ag+. In order to specifically measure ARSB activity, Pb2+ and Ag+ are added to the 4-MUS substrate buffer to favor and specifically observe ARSB activity in the presence of other sulfatases. The reaction-stop buffer has to contain EDTA to prevent the precipitation of Pb2+ carbonate (PbCO3 ) which may disrupt the fluorometric readings. Clinical signs due to enzyme deficiencies are generally observed when there is severe to complete deficiency and residual activities of less than 10% are thought to be cross-reactivities by related enzymes.3 The proband’s ARSB activity in leukocyte, liver and spleen was less than 5% of the normal control activity. This residual activity was likely non-specific ARSB-independent as the molecular studies predict a complete lack of ARSB protein (see below). Furthermore, the activity measured in vitro may not reflect the in vivo situation, as the conditions may not be optimal regarding substrate and acidic pH. A specific ARSB deficiency should also be differentiated from the multiple sulfatase deficiency, which is a progressive neurodegenerative lysosomal storage disorder that mainly affects the nervous system.6 Since no neurologic abnormalities were noted in the proband, this disorder could be dismissed based upon clinical signs.
It is well established that many mutations in dogs are very breed-specific. Indeed, this Great Dane puppy with MPS VI did not have one of the known mutations in the ARSB.1,18 However, exonic sequencing of ARSB revealed a homozygous nonsense point mutation (c.295C>T, p.Gln99*). This premature stop codon in exon 1 results in severe truncation of the protein at the equivalent of 98 amino acids instead of the wild-type length of 535 amino acids. However, degradation by nonsense-mediated mRNA decay, predicts no ARSB protein synthesis. The nonsense-mediated mRNA decay, a translation-coupled pathway employed by mammalian cells, allows the body to degrade truncated mRNAs before they are translated into nonfunctional polypeptides.25 The null mutation explains the severe phenotype of MPS VI in this Great Dane puppy. An identical mutation was previously found in human patients with severe MPS VI phenotype (c.289C>T, p.Gln97*), 19 but not in any animal models of MPS VI. However, the Toy Poodle18 and Miniature Schnauzers1,23 also have disease-causing mutations in exon 1 of ARSB and have a similar phenotype, whereas Miniature Pinschers1,22 with a missense mutation in exon 5 seem to have a milder phenotype.
While MPS VI in Miniature Schnauzers and Miniature Pinschers have been encountered in several dogs over the past decade, the Great Dane puppy of this report and the Toy Poodle from New Zealand appear to be isolated cases. The breeder has stopped breeding with the sire and has tested all offspring from an outcross of the dam with neutering of the single carrier produced. In a very limited survey, no other Great Danes were found to be carriers. Therefore, screening of Great Danes is not generally recommended unless other affected dogs are recognized.
Siamese cats were first recognized to have MPS VI.15,17 Interestingly one of the mutations in Siamese cats (c.1427T>C, p.L476P)31 shows a clinically severe phenotype while the other (c.1558G>A, p.D520N)30 results in a mild phenotype. Cats heterozygous for both mutations also show a mild phenotype (LA76P/D520N compound heterozygotes).9
Currently, there is no effective treatment or cure once clinical signs of this MPS VI disorder have developed in humans and animals, and the disease is progressive and fatal. Because MPS VI affects multiple body systems, management of a diverse spectrum of disease manifestations is a common and important part of providing supportive care to patients. Clinical management may include use of adaptive or supportive devices, physical and occupational therapy, symptom-based medications, surgical interventions, and therapies to provide the deficient enzyme.27 A more complete description of the affected systems with additional disease management information for humans can be found in the Management Guidelines for Mucopolysaccharidosis VI by Giugliani et al.13
Experimentally bone marrow transplantation, enzyme replacement and gene therapy in cats5,8,24,29 and humans4,20 are promising as long as the treatment is instituted neonatally prior to onset of severe clinical signs. However, these therapies have been less effective than predicted for murine models.10 Dogs and cats with naturally occurring MPS VI can be used as animal models in order to further understand the pathogenesis of MPS VI and for safety and efficacy assessment of novel therapeutic approaches in humans.
Supplementary Material
Acknowledgment
This study was supported in part by a grant from the National Institutes of Health (NIH OD 010939). The authors would like to acknowledge Dr. Lisa Olver at Justus Veterinary Clinic, Scott Twp, PA and Dr. Justin Nowowiejski at VCA Animal Specialty & Emergency Center, Wappingers Falls, NY for referring the case, collecting the specimens, and providing the radiographs and initial bloodwork.
Footnotes
Declaration of Conflicting Interests
Authors at the University of Pennsylvania are members of the non-for-profit PennGen Laboratories, which are offering metabolic and DNA testing for MPS and other hereditary storage and other disorders.
References
- 1.Berman L, Foureman P, Stieger K, et al. Mucopolysaccharidosis type VI caused by a point mutation in the miniature pinscher and deletion in the miniature schnauzer. 2nd International Conference: Advances in Canine and Feline Genomics Utrecht, The Netherlands, 2004; p. 60. [Google Scholar]
- 2.Berry HK. Screening for mucopolysaccharide disorders with the Berry spot test. Clin Biochem. 1987;20(5):365–371. [DOI] [PubMed] [Google Scholar]
- 3.Brooks DA, Gibson GJ, Karageorgos L, et al. An index case for the attenuated end of the mucopolysaccharidosis type VI clinical spectrum. Mol Genet Metab. 2005;85:236–238. [DOI] [PubMed] [Google Scholar]
- 4.Brunelli MJ, Atallah ÁN, da Silva EM. Enzyme replacement therapy with galsulfase for mucopolysaccharidosis type VI. Cochrane Database Syst Rev. 2016;4;3:CD009806. [DOI] [PubMed] [Google Scholar]
- 5.Byers S, Crawley AC, Brumfield LK, et al. Enzyme replacement therapy in a feline model of MPS VI: modification of enzyme structure and dose frequency. Pediatr Res. 2000;47:743–749. [DOI] [PubMed] [Google Scholar]
- 6.Chang PL, Rosa NE, Ballantyne SR, et al. Biochemical variability of arylsulphatases-A,-B and-C in cultured fibroblasts from patients with multiple sulphatase deficiency. J Inherit Metab Dis. 1983;6:167–172. [DOI] [PubMed] [Google Scholar]
- 7.Chang PL, Rosa NE, Davidson RG. Differential assay of arylsulfatase A and B activities: A sensitive method for cultured human cells. Anal Biochem. 1981;117:382–389. [DOI] [PubMed] [Google Scholar]
- 8.Crawley AC, Niedzielski KH, Isaac EL, et al. Enzyme replacement therapy from birth in a feline model of mucopolysaccharidosis type VI. J Clin Invest. 1997; 99:651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crawley AC, Yogalingam G, Muller VJ, et al. Two mutations within a feline mucopolysaccharidosist type VI colony cause three different clinical phenotypes. J Clin Invest. 1998;101:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferla R, Cotugno G, Claudiani P, et al. Gene therapy versus enzyme replacement therapy in a murine model of mucopolysaccharidosis VI. Mol Ther. 2012;S54–S55. [Google Scholar]
- 11.Fyfe JC, Kurzhals RL, Lassaline ME, et al. Molecular basis of feline beta-glucuronidase deficiency: an animal model of mucopolysaccharidosis VII. Genomics. 1999;58(2):121–128. [DOI] [PubMed] [Google Scholar]
- 12.García A, Niubò J, Benítez MA, et al. Comparison of two leukocyte extraction methods for cytomegalovirus antigenemia assay. J Clin Microbiol. 1996;34(1):182–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giugliani R, Harmatz P, Wraith JE. Management guidelines for mucopolysaccharidosis VI. Pediatrics. 2007;120:405–418. [DOI] [PubMed] [Google Scholar]
- 14.Haskins M, Giger U. Lysosomal storage diseases In: Kaneko JJ, Harvey JW and Bruss ML eds. Clinical Biochemistry of Domestic Animals. 6th ed. Burlington, USA: Elsevier Academic Press; 2008:731–749. [Google Scholar]
- 15.Haskins ME, Jezyk PF, Patterson DF. Mucopolysaccharide storage disease in three families of cats with arylsulfatase B deficiency: leukocyte studies and carrier identification. Pediatr Res. 1979;13:1203–1210. [DOI] [PubMed] [Google Scholar]
- 16.Hopwood JJ, Morris CP. The mucopolysaccharidoses. Diagnosis, molecular genetics and treatment. Mol Biol Med. 1990;7:381–404. [PubMed] [Google Scholar]
- 17.Jezyk PF, Haskins ME, Patterson DF, et al. Mucopolysaccharidosis in a cat with arylsulfatase B deficiency: a model of Maroteaux-Lamy syndrome. Science. 1977;198:834–836. [DOI] [PubMed] [Google Scholar]
- 18.Jolly RD, Hopwood JJ, Marshall NR. et al. Mucopolysaccharidosis type VI in a miniature poodle-type dog caused by a deletion in the arylsulphatase B gene. N Z Vet J. 2012;60(3):183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karageorgos L, Brooks DA, Pollard A, et al. Mutational analysis of 105 mucopolysaccharidosis type VI patients. Hum Mutat. 2007;28:897–903. [DOI] [PubMed] [Google Scholar]
- 20.Karageorgos L, Harmatz P, Simon J, et al. Mutational analysis of mucopolysaccharidosis type VI patients undergoing a trial of enzyme replacement therapy. Hum Mutat. 2004;23(3):229–233. [DOI] [PubMed] [Google Scholar]
- 21.Muenzer J The mucopolysaccharidoses: a heterogeneous group of disorders with variable pediatric presentations. J Pediatr. 2004;144(Suppl 5):S27–34. [DOI] [PubMed] [Google Scholar]
- 22.Neer TM, Dial SM, Pechman R, et al. Clinical vignette. Mucopolysaccharidosis VI in a miniature pinscher. J Vet Intern Med. 1995;9:429–433. [DOI] [PubMed] [Google Scholar]
- 23.Pérez ML, Kridel HA, Gallagher A, et al. Mucopolysaccharidosis type VI in a juvenile miniature schnauzer dog with concurrent hypertriglyceridemia, necrotizing pancreatitis, and diabetic ketoacidosis. Can Vet J. 2015;56(3):272–277. [PMC free article] [PubMed] [Google Scholar]
- 24.Ruane T, Haskins M, Cheng A, et al. Pharmacodynamics, Pharmacokinetics and biodistribution of recombinant human N-acetylgalactosamine 4-sulfatase after 6 months of therapy in cats using different IV infusion durations. Mol Genet Metab. 2016;117:157–163. [DOI] [PubMed] [Google Scholar]
- 25.Sardiello M, Palmieri M, di Ronza A, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325(5939):473–477. [DOI] [PubMed] [Google Scholar]
- 26.Shepard N, Mitchell N. Simultaneous localization of proteoglycan by light and electron microscopy using toluidine blue O. A study of epiphyseal cartilage. J Histochem Cytochem. 1976;24:621–629. [DOI] [PubMed] [Google Scholar]
- 27.Valayannopoulos V, Nicely H, Harmatz P, et al. Mucopolysaccharidosis VI. Orphanet J Rare Dis. 2010;5:5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang P, Sorenson J, Strickland S, et al. Mucopolysaccharidosis VII in a cat caused by 2 adjacent missense mutations in the GUSB gene. J Vet Intern Med. 2015;29(4):1022–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yogalingam G, Crawley A, Hopwood JJ, et al. Evaluation of fibroblast-mediated gene therapy in a feline model of mucopolysaccharidosis type VI. Biochim Biophys Acta. 1999;1453:284–296. [DOI] [PubMed] [Google Scholar]
- 30.Yogalingam G, Hopwood JJ, Crawley A, et al. Mild feline mucopolysaccharidosis type VI. Identification of an N-acetylgalactosamine 4-sulfatase mutation causing instability and increased specific activity. J Biol Chem. 1998;273:13421–13429. [DOI] [PubMed] [Google Scholar]
- 31.Yogalingam G, Litjens T, Bielicki J, et al. Feline mucopolysaccharidosis type VI. Characterization of recombinant N-acetylgalactosamine 4-sulfatase and identification of a mutation causing the disease. J Biol Chem. 1996;44:27259–27265. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida M, Noguchi J, Ikadai H, et al. Arylsulfatase B-deficient mucopolysaccharidosis in rats. J Clin Invest. 1993;91(3):1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.