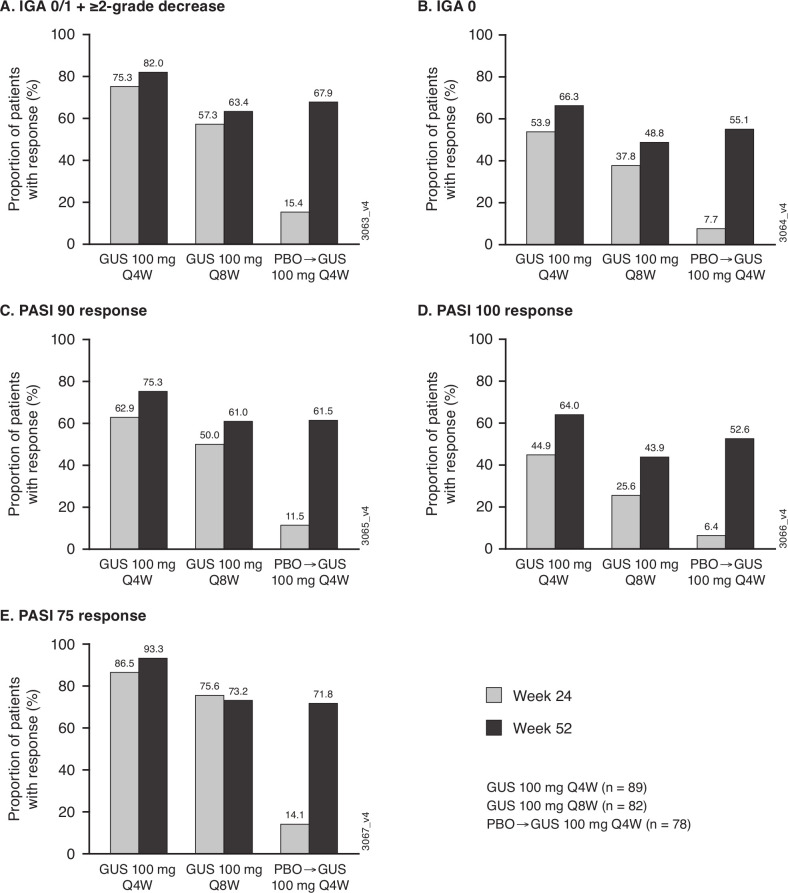
Figure 4.
Proportions of randomised and treated patients with ≥3% BSA psoriasis and IGA ≥2 at Week 0 achieving skin responses through Week 52. IGA 0/1 + ≥2-grade decrease from baseline (A), IGA 0 (B), PASI 90 (C), PASI 100 (D) and PASI 75 (E) response rates derived with application of data handling rules (see Methods). Previously reported Week24 data14 included for reference. Of 78 patients randomised to receive placebo who had ≥3% BSA psoriasis involvement and IGA ≥2 at baseline, 68 crossed over to guselkumab 100 mg Q4W (after Week24 response assessments); the 10 patients who received only placebo before discontinuing study agent were included as non-responders at Week52. BSA, body surface area; GUS, guselkumab; IGA, Investigator’s Global Assessment; PASI, Psoriasis Area and Severity Index; PBO, placebo; Q4/8W, every 4/8 weeks.