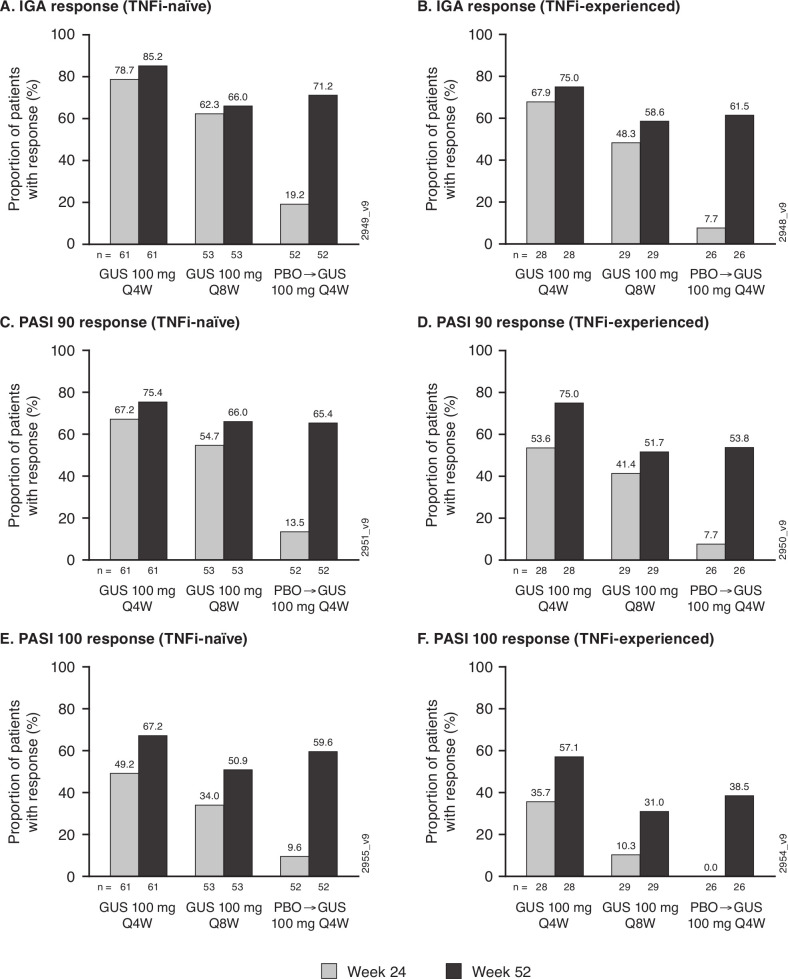
Figure 5.
Proportions of randomised and treated patients with ≥3% BSA psoriasis and IGA ≥2 at Week0 achieving IGA (IGA 0/1 +≥2-grade decrease from baseline), PASI 90 and PASI 100 responses at Week24 and Week52 by prior TNFi use. Panels (A), (C) and (E) summarise response in TNFi-naïve patients; panels (B), (D) and (F) summarise response in TNFi-experienced patients. Response rates derived with application of data handling rules (see Methods). Among 52 TNFi-naïve and 26 TNFi-experienced patients randomised to receive placebo, 47 and 21, respectively, crossed over to guselkumab 100 mg Q4W (after the Week24 response assessments); the remaining patients (five in each TNFi subgroup), who received only placebo and discontinued from the study, were included as non-responders at Week52. BSA, body surface area; GUS, guselkumab; IGA, Investigator’s Global Assessment; PASI, Psoriasis Area and Severity Index; PBO, placebo; Q4/8W, every 4/8 weeks; TNFi, tumour necrosis factor inhibitor.