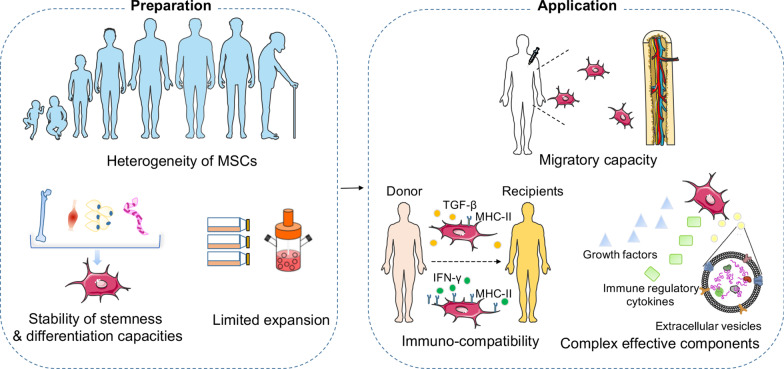
Fig. 2.
The main challenges in clinical applications of MSCs. During preparation of the MSC products, the main challenges include: (1) heterogeneity of MSCs resulted from donor variations such as the health status, genetics, gender, and age. (2) The varying degree of stability of stemness and differentiation capacities between MSCs isolated from different sources, such as bone marrow, adipose tissue, umbilical cord, or muscles. (3) The varying level of expansion capacities under different culture conditions, including confluence, culture surface, oxygen levels, flasks/bioreactors, passage number, and cell surface modifications. At the state of application, challenges remain due to the influence of (1) the homing or migratory capacity of MSCs under different administration route (local/systemic), injection site, infusion time, and cell carrier materials. (2) The immune compatibility between donors and recipients is the key to reduce the risk of rejection, but is affected by environmental inflammatory molecules which could induce distinct expression of MHC-II in MSCs. (3) The complex effective components released by MSCs depending on the host microenvironment (inflammation status, hypoxia, and ECM), which can result in highly variable factors shaping distinct functions of MSCs