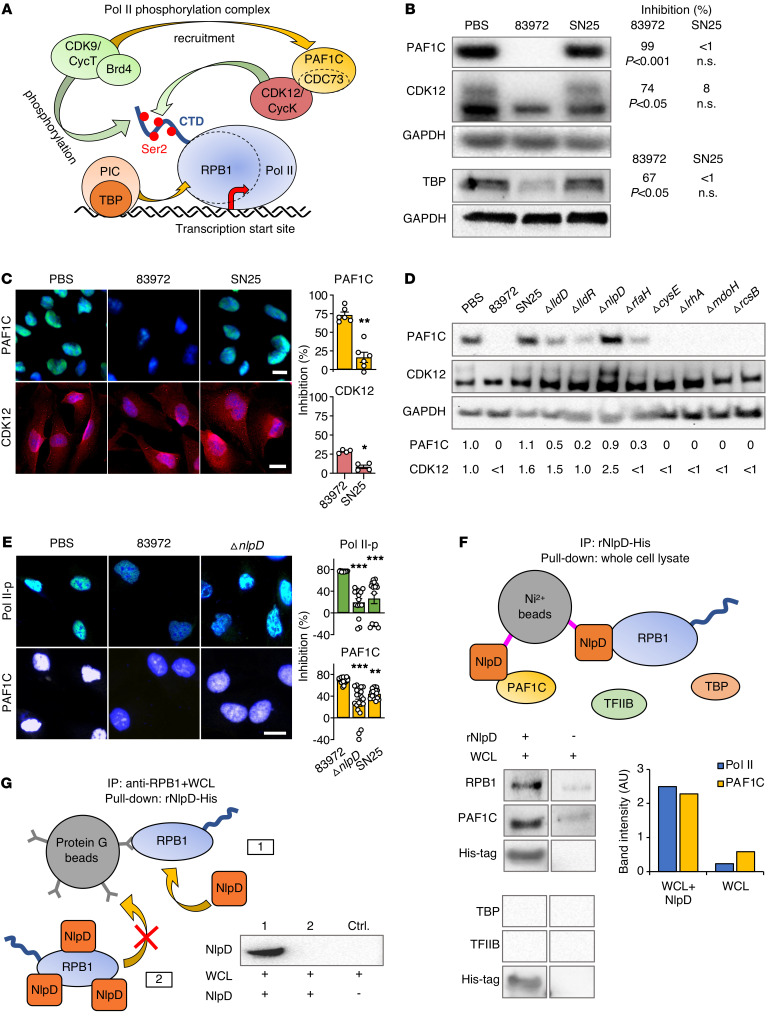
Figure 4. Effects on the Pol II phosphorylation machinery.
(A) Schematic of the Pol II phosphorylation machinery in eukaryotic cells (29). The Pol II phosphorylation complex binds to DNA at different eukaryotic promoters. CDK9 brings the PAF1C adaptor close to Pol II and the PAF1C subunit CDC73 recruits CDK12 to the complex. CDK9 and CDK12 then phosphorylate the Pol II subunit RPB1 CTD domain at Ser2. (B and C) PAF1C (CDC73) and CDK12 protein levels were markedly reduced by supernatants from E. coli 83972 but not E. coli SN25. (B) Western blot analysis of whole cell extracts, and (C) confocal imaging of human kidney cells. Histograms show quantification of fluorescence intensity. Data are presented as mean ± SEM (n = 4–5 experiments); Mann-Whitney U test. (D) Mutant screen for effects on PAF1C (CDC73) and CDK12 in human kidney cells. The ΔnlpD deletion mutant failed to inhibit PAF1C and CDK12. Western blot analysis of whole cell extracts. Band intensities were quantified (FC compared with PBS). (E) Loss of Pol II-p and PAF1C inhibition in E. coli SN25– and E. coli 83972 ΔnlpD–infected cells compared with those infected with E. coli 83972. Data are presented as mean ± SEM (n = 20 cells). Scale bars: 10 μm. *P < 0.05, **P < 0.01, ***P < 0.001 compared with E. coli 83972 by Kruskal-Wallis test with Dunn’s multiple-comparison test. (F) NlpD pull-down of RPB1 and PAF1C. Whole cell lysates (WCL) were exposed to Ni2+ beads coated with rNlpD-His and binding partners were identified by Western blot. TBP and TFIIB were not detected. (G) RPB1 binding to anti-RPB1–coated magnetic beads is inhibited by rNlpD (lane 2). Pull-down of RPB1 from whole cell lysates by the coated beads (lane 1) was competitively inhibited by rNlpD (lane 2). Data are representative of 3 independent experiments.