Scheme 3.
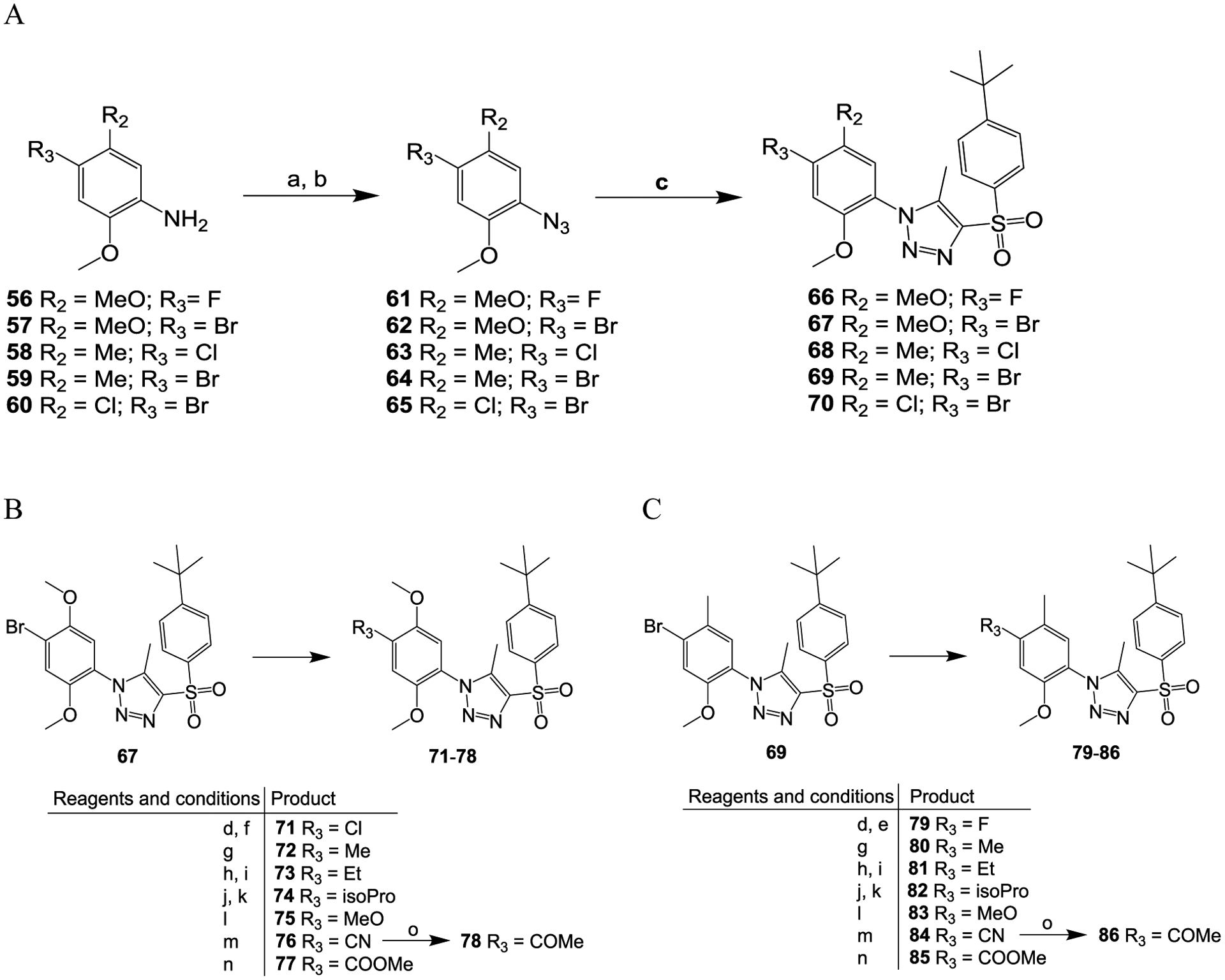
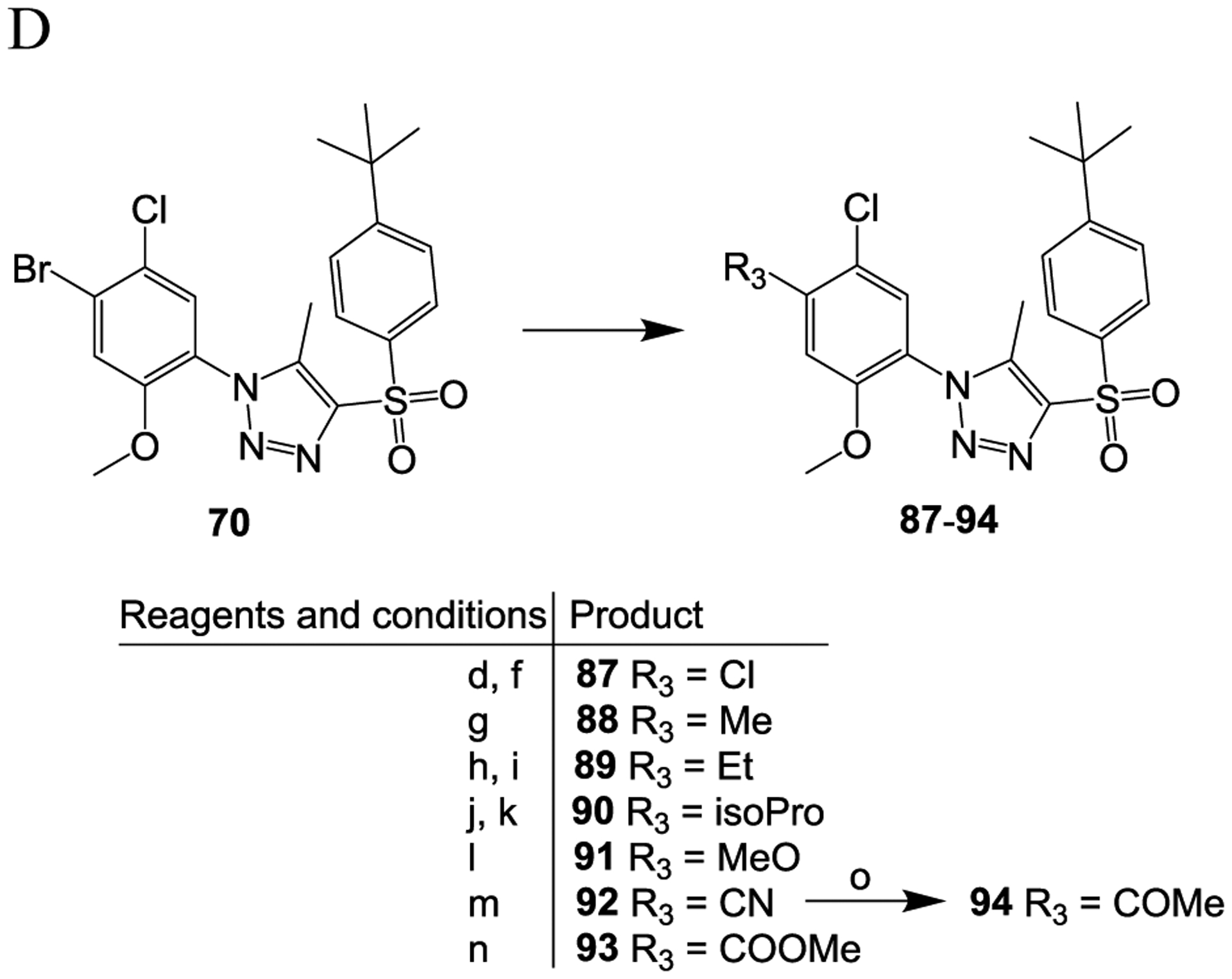
Reagents and conditions: (a) concentrated HCl, NaNO2, H2O, 0 °C, 15 min; (b) NaN3, H2O, room temperature, 2 h; (c) MeONa, 1-((4-(tert-butyl)phenyl)sulfonyl)propan-2-one, MeOH, 60 °C, overnight; (d) n-BuLi, THF, −78 °C, 5 min; (e) NFSI, THF, −78 °C, 1 h; (f) C2Cl6, THF, −78 °C, 1 h; (g) MeB(OH)2, Pd(PPh3)4, Na2CO3, dioxane, H2O, 120 °C, 5 h; (h) ethenylSn(Bu)3, Pd(PPh3)4, toluene, 120 °C, 5 h; (i) H2, 10% Pd/C, MeOH, THF, room temperature, 3 h; j) 4,4,5,5-tetramethyl-2-(prop-1-en-2-yl)-1,3,2-dioxaborolane, Pd(PPh3)4, Na2CO3, dioxane, H2O, 120 °C, 5 h; (k) H2, 10% Pd/C, MeOH, THF, room temperature, 3 h; (l) MeONa, CuCl2, DMF, 130 °C, overnight; m) CO (1.5 MPa), Pd(dppf)Cl2, MeOH, 80 °C, 24 h; (n) Zn(CN)2, Pd(dppf)Cl2, DMF, microwave, 160 °C, 15 min; (o) MeMgBr, THF, reflux, 15 h.
