Fig. 1 |. NADPH oxidase-dependent oxidase crosstalk in the pathogenesis of cardiovascular diseases.
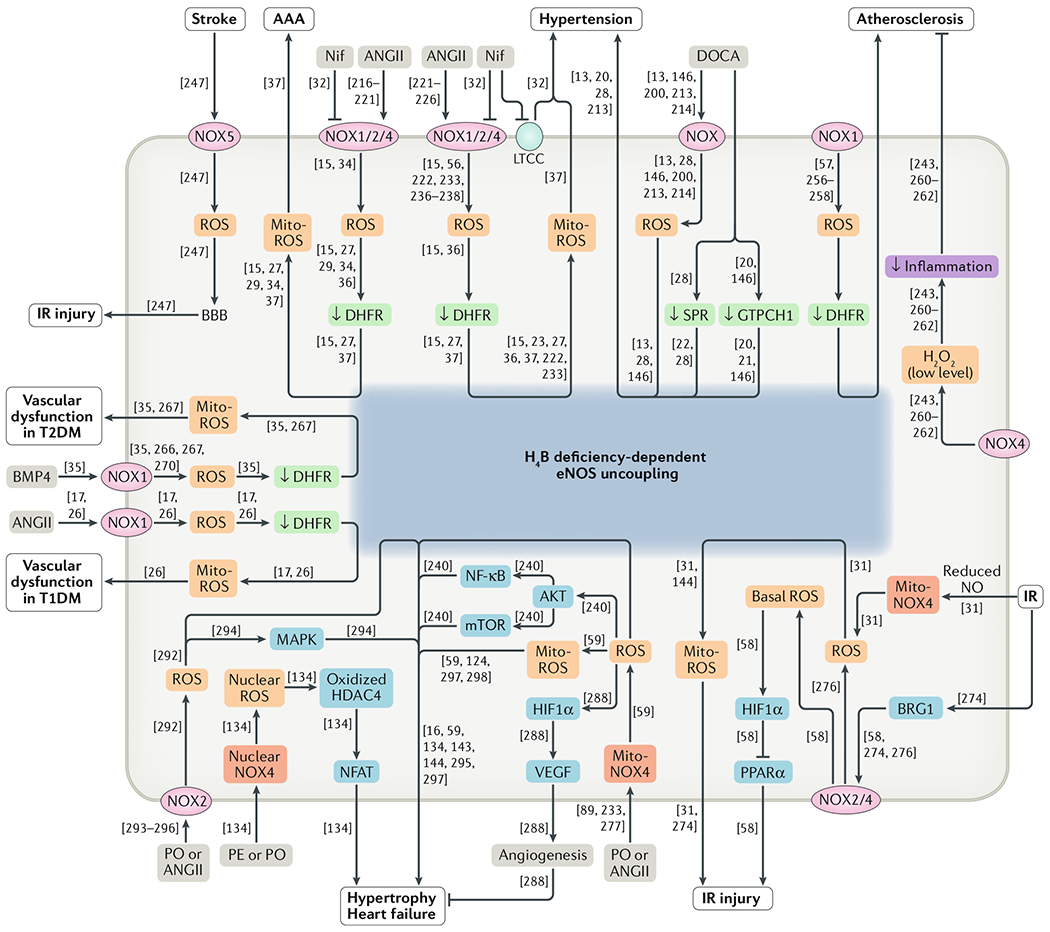
NADPH oxidase (NOX)-derived reactive oxygen species (ROS) production induces endothelial nitric oxide synthase (eNOS) uncoupling and mitochondrial dysfunction, resulting in sustained oxidative stress and the development of cardiovascular diseases. Reference numbers are given in square brackets. AAA, abdominal aortic aneurysm; AKT, RACα serine/threonine-protein kinase; ANGII, angiotensin II; BBB, blood–brain barrier; BMP4, bone morphogenetic protein 4; BRG1, transcription activator BRG1; DHFR, dihydrofolate reductase; DOCA, deoxycorticosterone acetate; GTPCH1, GTP cyclohydrolase 1; H2O2, hydrogen peroxide; H4B, tetrahydrobiopterin; HDAC4, histone deacetylase 4; HIF1α, hypoxia-inducible factor 1α; IR, ischaemia–reperfusion; LTCC, L-type calcium channel; MAPK, mitogen-activated protein kinase; Mito, mitochondrial; Mito-ROS, mitochondria-derived reactive oxygen species; mTOR, mechanistic target of rapamycin; NFAT, nuclear factor of activated T cells; NF-κB, nuclear factor-κB; N if, nifedipine; NO, nitric oxide; PE, phenylephrine; PO, pressure overload; PPARα, peroxisome proliferator-activated receptor-α; SPR, sepiapterin reductase; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; VEGF, vascular endothelial growth factor.
