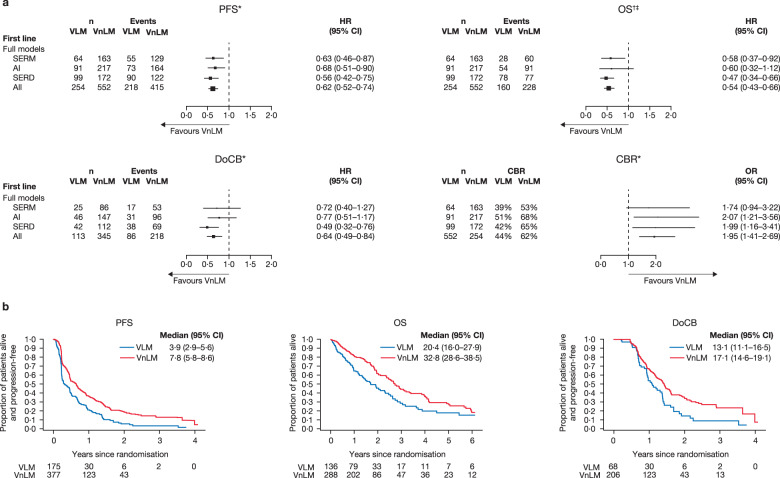
Fig. 3. Clinical outcome measures for VLM versus VnLM in the first-line setting.
a Forest plots of PFS, OS, DoCB and CBR. Data for VLM are not available for the EORTC trial. *Fixed effect for trial was fitted in all models. †Random effect for trial were fitted in model for AI. ‡OS data for Study 0027 are based on aggregated mature survival data. b Projected probability of PFS, OS and DoCB. Median (95% CI) PFS/OS/DoCB in months. AI aromatase inhibitor, CBR clinical benefit rate, DoCB duration of clinical benefit, HR hazard ratio, n number of patients, non-VM non-visceral metastases, PFS progression-free survival, OR odds ratio, OS overall survival, SERD selective estrogen receptor degrader, SERM selective estrogen receptor modulator, VLM visceral liver metastases, VM visceral metastases, VnLM visceral non-liver metastases. Statistics for full models: VLM vs VnLM PFS: SERM: p = 0·005, heterogeneity test p = 0·91; AI: p = 0·008, heterogeneity test p = 0·81; SERD: p < 0.001, heterogeneity test p = 0·95; All: p < 0·001, heterogeneity test p = 0·99. OS: SERM: p = 0·020, heterogeneity test p = 0·89; AI: p = 0·106, heterogeneity test p < 0·05; SERD: p < 0.001, heterogeneity test p = 0·34; All: p < 0·001, heterogeneity test p = 0·24. DoCB: SERM: p = 0·255, heterogeneity test p = 0·93; AI: p = 0·229, heterogeneity test p = 1·00; SERD: p < 0·001, heterogeneity test p = 0·08; All: p = 0·001, heterogeneity test p = 0·58. CBR: SERM: p = 0·077, heterogeneity test p = 0·72; AI: p = 0·008, heterogeneity test p = 0·43; SERD: p = 0·012, heterogeneity test p = 0·43; All: p < 0·001, heterogeneity test p = 0·80.