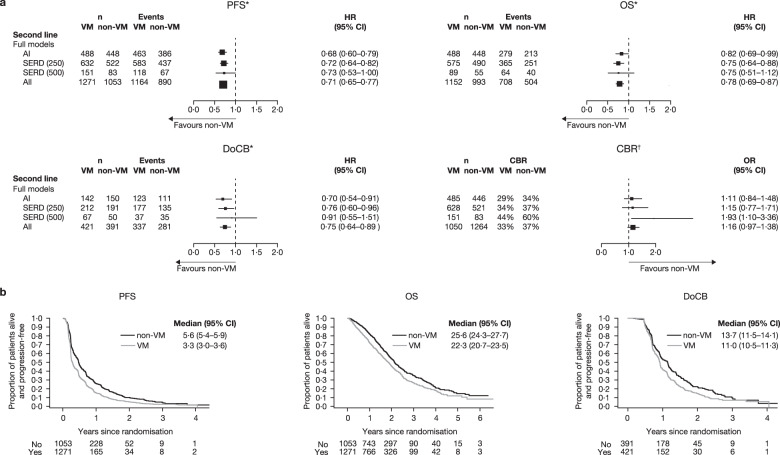
Fig. 5. Clinical outcomes for VM versus non-VM in the second-line setting.
a Forest plots of PFS, OS, DoCB and CBR. *Fixed effect for trial was fitted in all models. †Fixed-effect model was fitted to the SERD 250 mg, SERD 500 mg and all data; random effects for trial were included in the models for AI. b Projected probability of PFS, OS and DoCB. Median (95% CI) PFS/OS/DoCB in months. AI aromatase inhibitor, CBR clinical benefit rate, DoCB duration of clinical benefit, HR hazard ratio, n number of patients, non-VM non-visceral metastases, PFS progression-free survival, OR odds ratio, OS overall survival, SERD selective estrogen receptor degrader, SERM selective estrogen receptor modulator, VLM visceral liver metastases, VM visceral metastases, VnLM visceral non-liver metastases. Statistics for full models:PFS: AI: p < 0·001, heterogeneity test p = 0·13; SERD (250 mg): p < 0·001, heterogeneity test p = 0·08; SERD (500 mg): p = 0·048, heterogeneity test p = 0·83; All: p < 0·001, heterogeneity test p = 0·17. OS: AI: p = 0·036, heterogeneity test p = 0·35; SERD (250 mg): p < 0·001, heterogeneity test p = 0·95; SERD (500 mg): p = 0·161, heterogeneity test p = 1·00; All: p < 0·001, heterogeneity test p = 0·86. DoCB: AI: p = 0·007, heterogeneity test p = 0·40; SERD (250 mg): p = 0·023, heterogeneity test p = 0·08; SERD (500 mg): p = 0·720, heterogeneity test p = 0·31; All: p < 0·001, heterogeneity test p = 0·19. CBR: AI: p = 0·454, heterogeneity test p = 0·87; SERD (250 mg): p = 0·503, heterogeneity test p = 0·03; SERD (500 mg): p = 0·021, heterogeneity test p = 0·52; All: p = 0·109, heterogeneity test p = 0·10.