Abstract
Multiple studies and reports have suggested that coronavirus disease-19 (COVID-19) promotes arterial and venous thrombotic events in multiple organ systems, although the mechanism leading to a hypercoagulable state is still unknown. Few cases of splenic infarction associated with COVID-19 have been reported, of which half were found incidentally upon autopsy. This may be due to a clinically silent presentation or the symptoms being wrongfully attributed to pain caused by the effects of COVID-19. Due to the rarity of the condition and its lack of consistent symptomatology, splenic thromboembolism can be difficult to diagnose. Awareness of the condition and high clinical suspicion will help the clinician identify and manage the problem. Hemorrhage in patients with COVID-19 is uncommon in the hypercoagulable state that threatens thrombus formation in patients with COVID-19 infection. Despite prophylactic treatment with anticoagulation therapies, patients are more prone to developing clots. It is also well-known that therapeutic anticoagulation can place patients at a higher risk of bleeding. Thus, this unique population is at risk of developing both thrombotic and hemorrhagic events. We report a rare case of splenic infarction in a patient with confirmed COVID-19 infection despite prophylactic treatment with low-molecular-weight heparin which was found incidentally during workup for 2 other rare conditions: spontaneous rectus sheath hematoma and microhemorrhage or thrombus of the mesenteric vessels.
Keywords: COVID-19, Coronavirus, Sars-Cov-2, Splenic infarction, Rectus sheath hematoma
Introduction
In December 2019, the first cases of COVID-19 caused by the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) were reported in Wuhan, China. Since, there have been 95,612,831 confirmed cases of COVID-19, including 2,066,176 deaths, reported to the World Health Organization [1]. Multiple studies have been done to examine clinical and epidemiologic aspects of the disease [2], [3], [4], [5], [6], [7]. It is well established that COVID-19 has a myriad of presentations, ranging from asymptomatic to severe illness; the most severe cases being observed in the elderly and those with comorbid conditions [8,9]. COVID-19 predominantly attacks the pulmonary system, and causes acute lung injury and diffuse alveolar damage, but it also has been shown to affect multiple systems in patients with and without comorbidities. Literature suggests that infection with COVID-19 provokes arterial and venous thrombotic events [10], and could be contributing to the multisystem damage. In an autopsy study done by Falasca et al histopathological hallmarks of widespread vascular injury were found in the liver, kidney, bone marrow, and spleen [3]. The exact mechanism causing these findings is not yet understood, but it is theorized to be a distinct process unique to the Sars-Cov-2 virus [10]. The current postulations attribute the pathophysiological mechanisms of COVID-19-related hypercoagulopathy to systemic inflammatory response syndrome (SIRS) precipitated by cytokine storm or activation of the coagulation cascade due to cellular activation triggered by the virus, or both [11]. These pathologies are quantified by measurement of the D-dimer and fibrin degradation product levels which have been shown to correlate with the severity of disease and patient prognosis [7].
Interestingly, the patient we present suffered one confirmed and one possible hemorrhagic event as a result of being anticoagulated to prevent further thrombus formation. 80 mg prophylactic LMWH was being administered twice daily, but the measured D-dimer remained markedly elevated. COVID-19-related hypercoagulopathy dictates that clinically-overt bleeding is uncommon in the setting of COVID-19 [12]. However, bleeding is a risk of therapeutic anticoagulation. Our patient spontaneously developed bilateral rectus sheath hematomas (RSH) as well as mesenteric vessel microhemorrhage. Rectus sheath hematoma is an uncommon cause of acute abdomen and can be benign, but in the setting of a patient receiving anticoagulation agents, can quickly progress to a life-threatening event. To date, there is only one other case reported of rectus sheath hematoma in a patient with COVID-19 [13]. Splenic infarct, mesenteric vessel infarct, and rectus sheath hematoma are rare in COVID-19 patients and providers should be aware of these potential complications. We present a case where all 3 are concurrently present in the same patient.
Narrative
A male in his 70s with a past medical history of hypertension, benign prostatic hypertrophy, gastroesophageal reflux disease, and depression presented to the emergency department with a chief complaint of worsening dyspnea. Seven days prior, the patient developed a dry cough with sore throat, and subsequently tested positive for COVID-19 which he had been managing at home on an outpatient basis. Since his COVID-19 diagnosis, his symptoms continued to worsen, and upon arrival to the emergency department he had dyspnea at rest, nonproductive cough, fevers, nausea, decreased appetite, and weakness. He was found to be in acute hypoxic respiratory failure with oxygen saturation (O2 SAT) of 68% on room air. His O2 SAT increased to 88%-92% on a 15% nonrebreather mask. He was also tachypneic at 22 breaths per minute and afebrile at 37.6 °C. Chest X-ray revealed new extensive patchy consolidative opacities about the lungs, favored to represent multifocal pneumonia. Laboratory evaluation was significant for elevated D-dimer, fibrinogen, and ferritin at 14.41 mg/L, 620 mg/dL and 1973 mg/mL, respectively. Additionally, lactic acid and white blood cell count were both elevated at 2.6 mmol/L and 14,900 cells/uL, respectively. Blood and urine cultures were negative. Treatment with enoxaparin, dexamethasone, and remdesivir was initiated as the patient was admitted to the intensive care unit for inpatient management of sepsis, viral pneumonia, severe COVID-19 infection, and acute hypoxic respiratory failure.
On hospital admission day 2, repeat D-dimer continued to be elevated at 5.56 ng/mL. A duplex ultrasound examination of the bilateral lower extremities was performed which revealed no evidence of intraluminal thrombus. Over the course of the next few days, the patient reported improved symptomatology and resolved dyspnea. His acute hypoxic respiratory failure was improving; he was reduced from 90% FiO2 on BiPAP to 45% FiO2, high-flow nasal cannula/BiPAP. His care was transferred from intensive care to intermediate care. His D-dimer continued to be elevated at 4.20, 3.78, and 4.58 ng/mL on hospital admission days 3, 4, and 5, respectively.
On the morning of hospital admission day 6, the patient continued to endorse improvement of symptoms, including no nausea, vomiting, fever, or chills, but he began to complain of constipation. To manage said constipation, the patient was given 30 mL oral lactulose for symptomatic relief.
Later that evening, the patient had a large bowel movement. Subsequently, the patient experienced acute onset of severe left lower quadrant abdominal pain. CT scan of the abdomen and pelvis with intravenous contrast revealed several findings. A 4 cm well-demarcated area of nonenhancement within the anterior superior spleen (Fig. 1) consistent with acute infarct was seen. Additionally, there was inflammation within fat surrounding the mesenteric vessels in the left upper quadrant (Fig. 2) which was suspected to be microhemorrhage or thrombosis. There were also large hematomas within the bilateral rectus muscles (Fig. 3, Fig. 4, Fig. 5) beginning just above the umbilicus extending down to the pubis measuring 5.6 cm × 18 cm on the left (Fig. 4) and 7 cm × 4 cm × 10 cm on the right (Figs. 4 and 5). Contrast within the hematomas suggested active bleeding at the time of imaging (Fig. 3). Hemoglobin was tested and was found to have decreased from 13.7 g/dL at the time of admission to 10.7 g/dL shortly after the time of CT scan. The patient's full dose enoxaparin, which was initially started due to significantly elevated D-dimer, was subsequently discontinued due to the presence of actively bleeding hematomas, despite the presence of a splenic infarct. When measured earlier that morning, his D-dimer continued to be elevated at 3.90 mg/mL.
Fig. 1.
Axial CT image of the abdomen demonstrating a 4 cm well-demarcated area of nonenhancement within the spleen on abdominal CT with IV contrast.
Fig. 2.
Coronal abdominal CT image of the abdomen/pelvis demonstrating edema and fat stranding surrounding the left upper quadrant mesenteric vessels (red oval). This was thought to be due to mesenteric vessel microthrombi or hemorrhage. A definitive diagnosis was never established.
Fig. 3.
Axial CT image of the abdomen/pelvis demonstrating large hematomas within the bilateral rectus muscles (red arrows). Contrast within the left hematoma (red arrowhead) suggests active bleeding at the time of imaging. Dense fluid within the pelvis and along intrapelvic fascial planes is consistent with hemorrhage.
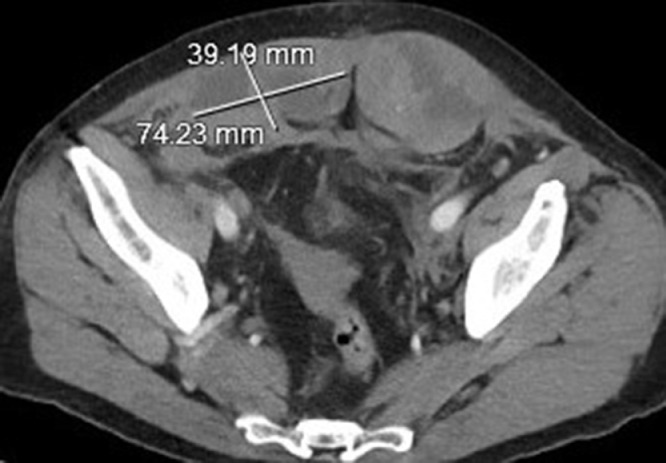
Fig. 4.
Sagittal CT images of the abdomen/pelvis with length measurement of the right and left rectus sheath hematomas.
Fig. 5.
Axial CT image of the abdomen/pelvis with diameter measurements of the right rectus sheath hematoma.
At this time, the decision was made to transfer the patient by helicopter to a tertiary care center for possible interventional radiology embolization as well as management of the large rectus hematomas. At this point, the patient had received 6 doses of IV remdesevir out of 10 as well as 6 days of IV dexamethasone. The patient was stable at the time of transfer.
After 2 days of in-patient care at the tertiary center, it was determined that the active bleeding had stopped, and the hematomas remained stable. The patient's COVID-19 symptoms had also improved, and he was able to maintain adequate oxygenation levels while ambulating. The patient was discharged to complete his recovery at home. His recovery course has gone well without complications and as of 2 months following discharge he has been able to safely return to normal activity.
Discussion
COVID-19 infection is well known to cause hypercoagulability with pulmonary emboli being the most common presentation, splenic artery embolism and splenic infarction are rarely reported [14], [15], [16]. Splenic infarct is a rare cause of abdominal pain, often secondary to a hypercoagulable state [7]. COVID-19 hypercoagulability has been proposed to occur due to elevation of proinflammatory cytokines, including IL-6 [14], [15], [16]. Additionally, elevated D-dimer and fibrinogen degradation products are associated with poorer disease prognosis, potentially related to risk of disseminated intravascular coagulation [16]. Thus, treatment of the hypercoagulable state with antithrombotic agents is appropriate.
In a brief literature review, we identified 5 case studies consisting of 6 patients with involvement of splenic thromboembolism secondary to COVID-19 infection, including one case of atraumatic splenic rupture, and one case of hemoperitoneum [7,10,[17], [18], [19]. Additionally, multiple post-mortem autopsies have identified splenic involvement secondary to COVID-19 infection [2], [3], [4], [5], [6]. There have also been 2 cases of psoas hematoma [20,21]. As of yet, there has been only one prior report of rectus sheath hematoma secondary to COVID-19 coagulopathy [13].
Abdominal scans are not routinely performed in COVID-19 patients as primary symptomatology involves the respiratory tract. Thus, only symptomatic splenic infarctions or those found incidentally on CT scans of the chest extending into the abdomen may be identified. Evidence of splenic involvement has been noted on autopsy in patients known to have had COVID-19 [2], [3], [4], [5], [6]. This suggests that the presence of splenic involvement due to COVID-19 hypercoagulability may be higher than reported. While most cases of splenic infarct may be asymptomatic, atraumatic splenic rupture can be a devastating complication and necessitates a high clinical index of suspicion for patients with abdominal pain and concurrent or prior COVID-19 infection.
Rectus sheath hematoma is an uncommon complication of anticoagulation therapy. Other risk factors include old age, female gender, history of abdominal surgery/trauma/injections, cancer, coagulopathies, and renal impairment [22]. We hypothesize that the development of RSH in our patient was due to shearing of the epigastric vessels caused by the combination of anticoagulation therapy and trauma caused by straining and/or coughing. Early diagnosis and intervention are key to improving patient mortality and morbidity. CT scan with IV contrast is considered the gold standard [22], for identification of bleeding and for differentiating between arterial and venous bleeds [13] In cases where conservative management is unsuccessful, or the patient presents with severe clinical criteria, CT angiography can be utilized to identify active bleeding and help in staging for interventional radiology treatment [23]. In patients who have contraindication to contrast, Doppler ultrasound and red cell scintigraphy can be used. The required treatment should be dictated by the severity of the RSH and status of the patient. Conservative therapy including a binder, rest and analgesics may be adequate in the stable patient, whereas patients with hemodynamic instability may require resuscitation using IV fluids and blood products. In patients who have been anticoagulated, intravascular coil embolization or rarely surgery to ligate the epigastric vessels, may be required to achieve adequate hemostatic control.
Conclusion
Splenic infarction in COVID-19 infection is rarely reported and may go undetected if symptoms are vague or obscured by other ailments such as constipation, placing patients at risk of splenic rupture. Likewise, SRSH are also rare, a source for abdominal pain and a potentially serious condition especially in the anticoagulated patient, necessitating emergent treatment and the need to weigh the risks of hemorrhage versus thrombus when considering anticoagulation reversal. Despite their rarity, these conditions are treatable and should be considered in COVID-19 patients with abdominal pain and radiologists should monitor for thrombosis to aid in early diagnosis. Additionally, further research or reports on acute hemorrhage in COVID-19 patients receiving anticoagulation is recommended.
Learning points
-
•
COVID-19 is well known to predispose patients for hypercoagulable events and complications, including thrombosis of unusual locations such as splenic artery branches.
-
•
Anticoagulant management of acute thrombotic events in COVID-19 is critical in prevention of morbidity and mortality.
-
•
Full anticoagulation predisposes patients to adverse effects related to bleeding and hemorrhage, including rectus sheath hematoma.
-
•
There are no clear guidelines on management of hemorrhagic events in patients on therapeutic anticoagulation as treatment of ischemia due to thrombus, the risks of hemorrhage versus thrombosis must be weighed.
-
•
Further research or reports on acute hemorrhage in COVID-19 patients receiving anticoagulation is recommended.
Patient consent statement
We are using entirely anonymized images from CT scans. These do not contain any identifying marks and are not accompanied by text that might identify the individual concerned.
Credit author statement
Jennifer Dennison: Writing-Original draft preparation and Editing, Investigation, Visualization: Samuel Carlson: Writing-Original draft preparation and Editing: Shannon Faehling: Writing-Original draft preparation and Editing: Hannah Phelan: Writing-Review and Editing: Muhammad Tariq: Writing-Review and Editing Ateeq Mubarik: Writing-Review and Editing, Supervision.
Footnotes
Institutional review board statement: Institutional Review Board approval was not necessary for our report.
Funding: None. The authors did not receive grant or outside funding in support of their research or preparation of this manuscript. They did not receive payment or any benefits from commercial entities.Declaration of competing interest
All authors declare that they have no competing interests.
References
- 1.WHO Coronavirus disease (COVID-19) Dashboard, World Health Organization Web site. https://covid19.who.int/. Published 2020. Accessed January 21, 2021.
- 2.Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet North Am Ed. 2020;396(10247):320–332. doi: 10.1093/infdis/jiaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falasca L, Nardacci R, Colombo D, Lalle E, Caro AD, Nicastri E. Postmortem findings in Italian patients with COVID-19: a descriptive full autopsy study of cases with and without comorbidities. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa578. https://www.thelancet.com/pdfs/journals/lancet/PIIS0140-6736(20)31305-2.pdf. Accessed January 10, 2021. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buja L.M., Wolf D.W., Zhao B., Akkanti B., McDonal M., Lelenwa L. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020 doi: 10.1016/j.carpath.2020. https://www.sciencedirect.com/science/article/pii/S1054880720300375. Accessed January 10, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X, Chang XN, Pan HX, Su H, Huang B, Yang M. [Pathological changes of the spleen in ten patients with coronavirus disease 2019(COVID-19) by postmortem needle autopsy] Zhonghua Bing Li Xue Za Zhi. 2020;49(6):576–582. doi: 10.3760/cma.j.cn112151-20200401-00278. Chinese. 2020 Jun 8. [DOI] [PubMed] [Google Scholar]
- 6.Duarte-Neto A.N., de Almeida Monteiro R.A., da Silva L.F.F., Malheirosa D.M.C., de Oliveira E.P., Filho J.T. Pulmonary and systemic involvement of COVID-19 assessed by ultrasound-guided minimally invasive autopsy. Histopathology. 2020;77(2):186–197. doi: 10.1111/his.14160. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agha OQ, Berryman R. Acute splenic artery thrombosis and infarction associated with COVID-19 disease. Case Rep Crit Care. 2020;2020:1–4. doi: 10.1155/2020/8880143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H. Clinical and immunological features of severe and moderate coronavirus disease-2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pessoa M.S.L., Lima C.F.C., Pimentel A.C.F., Costa Júnior J.C.G., Holanda J.L.B. Multisystemic infarctions in COVID-19: focus on the spleen. Eur J Case Rep Intern Med. 2020;7(7) doi: 10.12890/2020_001747. 001747. https://europepmc.org/article/med/32665933. Accessed January 10, 2021. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oudkerk M, Büller HR, Kuijpers D, van Es N, Oudkerk SF, McLoud TC. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the National Institute for Public Health of the Netherlands. Radiology. 2020 doi: 10.1148/radiol.2020201629. https://www-ncbi-nlm-nih-gov.proxy.lib.mcw.edu/pmc/articles/PMC7233406/ Accessed January 10, 2021. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.031. Accessed January 10, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakirov I., Bakirova G., Albalawi Y., Asiri A.Y., Faqihi F., Bairli I. Left Inferior epigastric artery injury in COVID-19 patient. Case report and literature review. Int J Surg Case Rep. 2020;76:415–420. doi: 10.1016/j.ijscr.2020.09.198. DOI: 10.1016j.ijscr.2020.09.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020:3–6. doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E, Dei Poli M, Resta M. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18(7):1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han H., Yang L., Liu R., Lui F, Wu K, Li J. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58(7):1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 17.Shaukat I, Khan R, Diwakar L, Kemp T, Bodasing N. Atraumatic splenic rupture due to covid-19 infection. Clin Infect Pract. 2020 doi: 10.1016/j.clinpr.2020.100042. 100042. https://pubmed.ncbi.nlm.nih.gov/32999997/. Accessed January 10, 2021. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hossri S., Shadi M., Hamarsha Z., Schneider R., El-Sayegh D. Clinically significant anticardiolipin antibodies associated with COVID-19. J Crit Care. 2020 doi: 10.1016/j.jcrc.2020.05.017. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7256550/ Accessed January 10, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karki S., Rawal S.B., Malla S., Rayamajhi J., Thapa B.B. A case report on spontaneous hemoperitoneum in COVID-19 patient. Int J Surg Case Rep. 2020;74:211–213. doi: 10.1016/j.ijscr.2020.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel I., Akoluk A., Douedi S., Upadhyaya V., Mazahir U., Costanzo E. Life-threatening psoas hematoma due to retroperitoneal hemorrhage in a COVID-19 patient on enoxaparin treated with arterial embolization: a case report. J Clin Med Res. 2020;12(7):458–461. doi: 10.14740/jcomr4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo S.H., Zhu S.M., Yao Y.X. Giant retroperitoneal hematoma during extracorporeal membrane oxygenation in a patient with coronavirus disease-2019 pneumonia. J Cardiothorac Vasc Anesth. Oct 2020;34(10):2839–2840. doi: 10.1053/j/jvca.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of risk factors for rectus sheath hematoma. Clin Appl Thromb Hemost. Apr 2016;22(3):292–296. doi: 10.1177/1076029614553024. [DOI] [PubMed] [Google Scholar]
- 23.Pierro A, Cilla S, Modugno P, Centritto EM, De Filippo CM, Sallustio G. Spontaneous rectus sheath hematoma: the utility of CT angiography. Radiol Case Rep. 2018;13(2):328–332. doi: 10.1016/j.radcr.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]